ORIP Director’s Blog
The Director's Blog is written by the ORIP Director, Dr. Franziska Grieder. The blog highlights ORIP priorities, important research, and upcoming events. Learn more below.
Winter 2025
ORIP Strategic Plan 2026–2030
I am pleased to announce the release of the ORIP Strategic Plan 2026–2030, which outlines a forward-looking vision for research infrastructure that supports nearly all NIH institutes and centers. Anchored firmly in the NIH mission, the plan highlights emerging needs, including improved rigor and reproducibility and integration of new approach methodologies. The plan underscores the importance of adaptable and future-ready infrastructure that drives innovation with the goal of advancing human health.
Thank you all for your contributions!
Fall 2025
ORIP’s S10-Funded Instruments Support FDA Approval of New Alzheimer’s Disease Drug
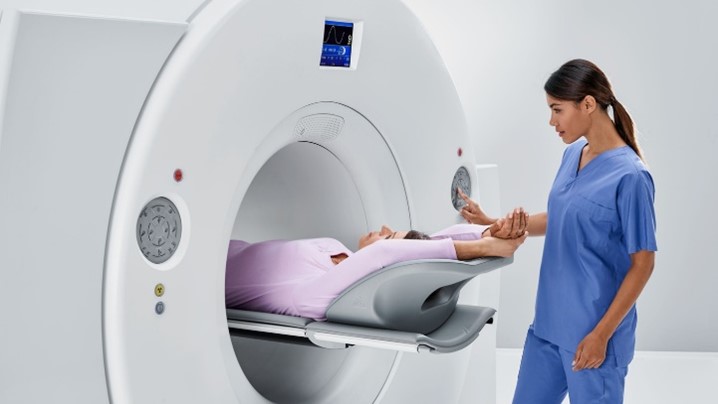
Alzheimer’s disease, the leading cause of dementia among older adults, is estimated to affect more than 6 million people in the United States. For decades, the underlying cause of Alzheimer’s disease has remained elusive to researchers, and confirmed cases could only be diagnosed by brain biopsy.
A leading research scientist in the efforts to improve Alzheimer’s diagnostics and treatment is Dr. Tammie Benzinger, the Hugh Monroe Wilson Professor of Radiology and Chief of Magnetic Resonance Imaging (MRI) Service at the Mallinckrodt Institute of Radiology at Washington University (WashU) School of Medicine in St. Louis. Dr. Benzinger and her team use cutting-edge MRI and positron emission tomography/computed tomography (PET/CT) scanning to study biomarkers associated with neurodegenerative diseases, including Alzheimer’s disease.
Much of this work is conducted at WashU’s Center for Clinical Imaging and Research (CCIR), a centralized, hospital-based research facility. Among the CCIR’s imaging tools are two Siemens Biograph Vision 600 PET/CT scanners. One of these scanners was acquired in 2018 with support from the Office of Research Infrastructure Programs (ORIP) through an S10 Shared Instrumentation Grant. PET/CT scanners are essential for clinical research, but their high cost often limits access. ORIP’s funding makes this technology available to a broader research community at the university.
This S10-funded scanner played a key role in a clinical trial testing the safety and effectiveness of donanemab, an antibody treatment aimed at clearing amyloid plaques in individuals with early symptomatic Alzheimer’s disease. The trial’s findings supported the U.S. Food and Drug Administration (FDA) approval of donanemab in July 2024.
Dr. Benzinger and her colleagues were among the first to study Alzheimer’s disease using this high-end imaging technology. Shortly after acquiring the ORIP-supported scanner, the center secured additional funding to purchase a second identical system, enabling faster and more consistent data collection. In September 2024, the team expanded its capabilities even further with a new whole-body human 7-Tesla MRI scanner (MAGNETOM Terra.X), purchased through a second S10 award. Blood-based diagnostics are being evaluated against established PET imaging protocols using the same ORIP-funded scanners.
About ORIP’s S10 Instrumentation Programs
The journey from discovery to diagnosis and treatment depends on sustained investment in cutting-edge research infrastructure. ORIP’s Shared Instrumentation Grant Programs, which were acknowledged in 700 Alzheimer’s disease publications during the past 5 years, exemplify how federal support can catalyze scientific breakthroughs and directly contribute to life-changing clinical outcomes. As researchers like Dr. Benzinger continue to push the boundaries of what is possible in Alzheimer’s disease research and beyond, ORIP-funded instruments will remain vital to accelerating progress, expanding access to technology, and ultimately improving lives.
Spring 2025
Cryopreservation—A Vital Need

Animal models are indispensable for advancing our understanding of human diseases, developing treatments, and preparing for future public health challenges. Cryopreservation of animal tissue has long been essential for preserving endangered species, and its significance in biomedical research is profound and far-reaching.
Recognizing the importance of cryopreservation, ORIP sponsored a series of workshops in fall 2024 to explore the evolving needs and advancement of this field across various model organisms—including rodents, aquatic species, and invertebrates—as well as emerging cryopreservation technologies. These workshops convened leading domestic and international experts to assess current methodologies, identify challenges, and drive innovation in storage, reproductive viability, and biocompatibility.
Key discussions highlighted recent breakthroughs in rodent sperm cryopreservation, emphasizing its role in streamlining access to genetically valuable mouse models. The aquatic model cryopreservation workshop addressed protocols for sustainable repositories that can distribute genetic resources for use by the scientific community. The session on invertebrates underscored the substantial cost savings of cryopreserved samples compared to traditional animal husbandry. Meanwhile, discussions on cryopreservation technologies stressed the urgent need for biocompatible methods that allow long-term specimen storage without relying on cell-permeating agents or liquid nitrogen.
With more than 300 participants, these workshops demonstrated the strong demand for continued NIH investment in cryobiology. Cryobanking, particularly in rodent research, remains a cornerstone of laboratory efficiency, expediting scientific progress by making vital models more accessible. Through these ORIP-led efforts, the scientific community has gained deeper insights into cutting-edge cryopreservation strategies to enhance genetic resource management and distribution.
Ultimately, cryopreservation is not just a research tool: It is a critical component of NIH’s mission to advance human health and combat disease. Ensuring the sustainability and innovation of cryopreservation techniques will be essential for the future of biomedical research.
Franziska B. Grieder, DVM, PhD
Director, Office of Research Infrastructure Programs
National Institutes of Health
Department of Health and Human Services
Fall 2024
Shaping the Future: ORIP’s Strategic Plan for 2026–2030

At NIH, we develop Strategic Plans as a roadmap to describe our organization’s long-term vision, set the goals to achieve, and provide direction moving forward. Developing a Strategic Plan requires extensive and collaborative teamwork, and it must incorporate input from our stakeholders—grantees, applicants, broad scientific communities, and NIH partners. As we approach the close of 2024, I wanted to take the opportunity to provide an update on ORIP’s activities toward ORIP’s third strategic plan that will guide ORIP’s progress and success from 2026 through 2030.
In the summer of 2023, we launched the planning process with an office-wide retreat that brought together all ORIP members for a day of in-depth discussion and review. Interdisciplinary teams, each focused on one of ORIP’s strategic priorities, provided subject-matter expertise while incorporating diverse perspectives. This collaborative effort set the stage for refining our goals and identifying areas for growth. In January 2024, I presented an update to the Council of Councils ensuring this NIH advisory body was informed of ORIP’s progress and achievements. This transparent communication reinforced our commitment to excellence and accountability.
Over the past year, the ORIP teams have meticulously evaluated and refined our strategic priorities to ensure alignment with our mission: providing infrastructure nationwide to support research across all scientific disciplines. Our work emphasizes fostering collaborations, promoting transparency, ensuring rigor and reproducibility, and stewarding public investments. This process has been enriched by input from a broad spectrum of experts. Internal and external NIH subject-matter specialists offered insights, challenged assumptions, and helped shape future directions. For example, virtual focus groups with grantees informed our priorities for physical infrastructure, while broad and wide consultations with experts guided our vision for disease model development. These efforts reflect ORIP’s commitment to activities necessary to continuous program improvement, extending beyond the scope of the strategic plan itself.
With the ORIP teams fully engaged, I am confident that we will produce a strategic plan that serves as a beacon for our next five years. The next steps include refining the draft, integrating feedback, and finalizing details. This phase also offers creative opportunities, such as selecting cover art and vignettes that highlight ORIP’s impact. The cover will symbolize ORIP’s commitment to enabling innovation, while the vignettes will showcase how our resources drive health breakthroughs and discoveries. I am inspired by the dedication of ORIP’s staff and grateful for the invaluable contributions of our stakeholders and advisors. Together, we are shaping a strategic plan that will not only guide ORIP’s future but also amplify our impact on the broader scientific community. Wishing everybody a wonderful holiday season and a prosperous New Year.
Franziska B. Grieder, D.V.M., Ph.D.
Director of the Office of Research Infrastructure Programs
Summer 2024
A Thanksgiving in September

As fall approaches and a new school year begins, we also near the end of the federal fiscal year (FY), which runs from October 1 to September 30. For those of us in the federal sector, particularly in the Office of Research Infrastructure Programs (ORIP), September is a time of intense activity. It involves strategically allocating remaining funds and meticulously tracking the year’s accomplishments. These efforts are not merely procedural; they are essential to fulfilling our commitment to the American people.
Reflecting on ORIP’s achievements over the past year is a powerful reminder of the dedication and expertise that my colleagues bring to our work. Their commitment to excellence is evident in every accomplishment, underscoring the effect we collectively have on advancing biomedical research. Here are a few highlights that stand out from the many we could celebrate:
First, it is remarkable to note that this fiscal year, ORIP’s team of just nine program officers successfully managed more than 1,000 grants, many of which were highly complex. This level of efficiency and oversight is a testament to their exceptional skill and commitment.
Second, ORIP continues to excel in fostering collaboration across the National Institutes of Health (NIH) and beyond. By working with other NIH institutes, centers, offices, and programs, we have established more than 20 co-funding partnerships this year alone. These collaborations are not only driving innovative research but also enhancing our collective impact on public health. A standout example is our partnership with the NIH Office of AIDS Research (OAR) on the Nonhuman Primate Evaluation and Analysis: Final Report. This comprehensive report provides an in-depth analysis of the demand and supply for nonhuman primates in the United States, covering trends from FY18 to FY22, forecasting through FY28, and strategically informing NIH stewardship of critical nonhuman primate research resources.
Third, ORIP remains at the forefront of advancing rigorous and reproducible scientific research, particularly in the realm of animal models. Following the 2022 workshop Enhancing Rigor and Reproducibility in Animal Research by Managing Extrinsic Factors, we launched a new funding opportunity this year that aims to deepen our understanding of how extrinsic environmental factors influence animal research outcomes. Applications are expected in September.
I am consistently impressed by the unwavering commitment of ORIP’s program officers, analysts, and support staff to our mission and to driving forward scientific innovation. Their dedication and relentless pursuit of excellence are crucial to our success and to the advancement of biomedical research. Reflecting on the year’s successes not only reinforces the value of our work but also highlights the extraordinary efforts of an exceptional team that consistently delivers results. As we close in on the end of another fiscal year, I want to extend my gratitude to everyone who has contributed to our efforts. It is your dedication and hard work that drive our mission forward, and for that, I am deeply thankful.
Franziska B. Grieder, D.V.M., Ph.D.
Director of the Office of Research Infrastructure Programs
Winter 2024
ORIP Supported Critical Research Infrastructure and Technology During the COVID Pandemic
Four years ago, I and many of my NIH colleagues came to work to close down our offices and take whatever we needed to continue our work while remaining safely at home. To mark the passing of this day on March 15, 2020, I would like to take a moment to reflect on the role ORIP played in providing infrastructure and supporting COVID research then and now.
Throughout the crisis, ORIP staff evaluated and developed new management plans, preserved and maintained animal resources, continued research center operations to support biomedical communities, and built new resources for COVID research.
ORIP’s S10 grant provided shared instruments for NIH-funded investigators that are research-agnostic and were immediately put to use for COVID vaccine research. In 2020 alone, 77 S10-supported papers were published on COVID or SARS-CoV-2, 537 publications total to date have been published on this topic using S10 support. Of note are two papers that were published in 2020. A pixel array detector for macromolecular crystallography was awarded through an S10 grant in 2016 to a researcher at Cornell and was made available through the Advanced Photon Source. The instrument was the used by a group in Minnesota for their SARS-CoV-2 research that elucidated the structural basis of receptor recognition by the virus, which was published in Nature and has been cited 2,386 times to date. The other noteworthy S10 supported publication is a Science paper by investigators at the University of California, San Francisco focusing on neutralizing SARS-CoV-2 with synthetic nanobody. Both these studies contributed to our understanding of the structure and mechanism of the virus.
As the S10 program provides a way for core investigators to obtain access to emerging technologies, ORIP continues supporting the development of such technologies through its small business program. One of these small businesses, Combinati (now Thermo Fisher Scientific), created a highly innovative and easy-to-use digital PCR platforms. It offers absolute quantification of DNA, cDNA, or RNA. Digital polymerase chain reaction (dPCR) is a biotechnological refinement of conventional PCR methods that can be used to directly quantify and clonally amplify nucleic acids strands.
In the post-COVID world, we continue to see the benefits of infrastructure that can adjust to new research needs, such as long COVID (LC). LC covers a wide range of new, returning, or ongoing health problems following an acute COVID infection. It is not one illness, no test is available to diagnose it, the symptoms are hard to explain and manage, it affects different individuals in different ways, and clinical evaluations may even reflect a false negative. LC research is supported through several ORIP programs, such as administrative supplements to NPRCs for animal models to learn more about LC but is also researched using S10-provided instruments that have been funded long before the onset of the pandemic. As such, a recent publication in Nature Immunology used ORIP-funded Flow Cytometers, to analyze the characteristics of cells, examining T cell dysregulation in LC patients. In comparison to those who have recovered, the study revealed potential issues with communication between the cellular and humoral arms of the immune system. This breakdown in communication may result in immune dysregulation, inflammation, and the clinical symptoms associated with LC.
Although, the initial months of the pandemic are now behind us, the pandemic clearly illustrated to us the extraordinary benefit of having critical infrastructure and technology already in place to readily support the urgent, emerging, and sustaining research needs, such as vaccine discovery.
Franziska B. Grieder, DVM, Ph.D.
Director of the Office of Research Infrastructure Programs
Fall 2023
ORIP Research Results in Successful Pediatric Valve Transplant

ORIP-funded research resources serve as powerhouses to energize many areas of research. A recent advancement in the treatment of critical congenital heart defects (CCHDs) is one such area that has been benefited from ORIP-funded resources. CCHDs are particularly difficult to treat, with heart transplants being a last-resort cure. Besides the scarcity of donor organs, a major challenge for pediatric heart transplants is that the patient’s heart grows and soon outpaces the transplanted tissue, forcing patients to go through repeated life-threatening operations as they grow.
In 2022, a successful partial heart transplant of growing valves was performed on Owen Monroe, a 17-day-old low-birthweight boy with a rare fatal congenital heart condition, at the Duke University Pediatric Cardiac Surgery. This was hailed as a miraculous medical achievement. Six months after the world’s first partial heart transplant, Owen is healthy and showing no signs of needing a reoperation any time soon: his transplanted valve is growing with him. This success shows a great potential for partial heart transplant as a revolutionary treatment of critical congenital heart defects.
This accomplishment also highlights fundamental research conducted by the National Swine Resource and Research Center (NSRRC), one of the ORIP-funded resource centers. Using NSRRC’s newly developed green fluorescent transgenic swine models, researchers studied the destiny of transplanted cells by visualizing and tracking integration and/or differentiation of the transgenic cells after transplantation. With transgenic swine model and green fluorescent protein markers, they conducted phenotyping studies in pig-to-pig allotransplant models and pig-to-nonhuman-primate xenotransplant models, showing soundness of the partial heart transplantation technology (Bishara et al., Journal of Cardiovascular Development and Disease, 2023 June 10). Their studies prove that transplanted valves can indeed grow alongside their hosts, paving the way for clinical translation of partial heart transplant technology.
Franziska B. Grieder, DVM, Ph.D.
Director of the Office of Research Infrastructure Programs
Spring 2022
NPRC Scientific Seminar Series Marks 60 Years of NIH Support

One of ORIP’s flagship programs, the National Primate Research Centers (NPRCs), celebrate six decades of biomedical research advancements with a 60th Anniversary Seminar Series in spring 2022. First established by Congress in the 1960s as the Regional Primate Research Centers Program, the Centers expanded their reach to support the research community across the United States. As a result, they were renamed as National Primate Research Centers in 2001 to reflect their national and international impact on biomedical research and innovation. The NPRCs support many research disciplines by providing cutting-edge facilities, well-characterized nonhuman primate (NHP) models for research, and expertise in all aspects of NHP biology, husbandry, and research to scientists conducting basic and translational research.
To recognize 60 years of NIH support, the NPRC Consortium of seven Centers is sponsoring a virtual seminar series featuring both senior and early-stage investigators, who will share the latest research and scientific advancements made with NHPs. Physician-scientists will also participate in the series to provide the clinical perspective that helps inform future research directions.
The seven-part seminar series will run from April 27 to June 8, 2022. The first session will focus on HIV/AIDS, an area where studies using an SIV-infected macaque model led to the breakthrough antiviral therapies used today to control HIV infection in humans (April 27). Subsequent sessions will explore the role of NHPs in the following research areas:
- Developing treatments or vaccines for other infectious diseases (April 28);
- Addressing neurodegenerative disorders (May 11);
- Advancing behavioral and neurological research (May 12);
- Using genetic and stem cell technologies to treat diseases (May 25);
- Advancing reproductive biology and endocrinology research (May 26); and
- Investigating the impact of genomic variation on NHP research (June 8).
Join us in celebrating the NPRCs through these free, virtual seminars. (Registration has closed.)
NPRCresearch.org offers comprehensive information for investigators, collaborators, NIH program officials, and the public regarding the NPRCs’ areas of research, capabilities, NHP species, and achievements. The NPRC.org website focuses on educating the public about the benefits of NHP research. To learn more about the NPRCs visit: ORIP National Primate Research Centers Consortium.
Franziska B. Grieder, DVM, Ph.D.
Director of the Office of Research Infrastructure Programs
Winter 2022
Progress on ORIP Strategic Priorities
To mark one year since releasing the ORIP Strategic Plan 2021–2025, we are happy to announce the launch of the ORIP Strategic Plan Progress Update webpages. These pages describe progress in our research infrastructure high-priority thematic areas:
- Animal Models to Advance the Study of Human Disease
- Innovative Instruments and Equipment to Accelerate Research Discoveries
- Specialized Research Training in Animal Models and Related Resources
- Awareness of ORIP Resources and Programs
ORIP funds the scientific human and physical resources that will help to ensure the Nation’s capability to adapt to scientific challenges and opportunities. Since the release of ORIP’s Strategic Plan in January 2021, ORIP-funded scientists and staff have been working to make the research goals and objectives a reality. Examples of ORIP’s ongoing progress will be added to the website quarterly.
For more in-depth stories of ORIP-supported research and resources, check out ORIP’s monthly Research Highlights. These stories celebrate ORIP-funded researchers, display how ORIP-funded instruments propel research progress, and showcase how animal models offer insight into human diseases. For the most recent installment, start with A New Method for Drosophila Cryopreservation Overcomes Longstanding Challenges.
As ORIP progresses with strategic Theme 4: Awareness of ORIP Resources and Programs, ORIP will offer more webinars and other informational resources to support its applicants and grantees in all the scientific and training areas ORIP supports. For its next webinar, on Friday, February 18, 2022, 2:00 p.m.-4:00 p.m., ORIP staff will provide a Pre-Application Webinar for PAR-22-088, Biomedical Research Facilities (C06 Clinical Trial Not Allowed) and PAR-21-363, Limited Competition: Development and Renovation of Housing, Breeding, and Research Spaces for Existing NIH-supported NHP Colonies (C06 Clinical Trial Not Allowed). ORIP staff members involved in managing these programs will assist potential applicants by explaining the goals and objectives of these Funding Opportunities and answering questions from attendees. Learn more about the webinar at NOT-OD-22-060. Registration is available here.
The ORIP website has many resources to keep you in the know about our funding opportunities, resources, and research progress. Check back to find out the latest, especially for our continued strategic plan progress.
Franziska B. Grieder, DVM, Ph.D.
Director of the Office of Research Infrastructure Program
Fall 2021
ORIP Support for Modern Equipment for Shared-Use Biomedical Research Facilities

Welcome to fiscal year 2022 and the inaugural Director’s Message from the National Institutes of Health (NIH) Office of Research Infrastructure Programs (ORIP). As many of you know, ORIP funds scientific, human, and physical resources to ensure the Nation’s capability to prevent, diagnose, and treat human disease. In this first ORIP Director’s Message, we are pleased to announce the launch of our new Resource-Related Research Projects (R24) grant program: “Modern Equipment for Shared-use Biomedical Research Facilities: Advancing Research-Related Operations” (PAR-21-326).
Through discussions with grantees, applicants, and scientific researchers from a broad range of biomedical investigations, ORIP identified a need to provide support for equipment that is not used directly for scientific data collection but enhances the operation of biomedical research facilities. In addition to providing for such equipment, the new program covers minor alteration and renovation that might be needed to ensure proper installation and functioning of the equipment.
This initiative targets core facilities, animal research facilities, and other research spaces that are used on a shared basis. The program is intended to introduce operational innovations or new capacities rather than be used for general outfitting, routine maintenance, or simply replacing older equipment. By providing access to modern shared-use equipment through this new funding opportunity, we expect that the scientific community can expand their capacity for essential support services to evolving and emerging research programs.
The Modern Equipment for Shared-use Biomedical Research Facilities program is managed by ORIP’s Division of Construction and Instruments (DCI) which supports improvements of research infrastructure by enabling access to state-of-the-art shared instruments and by modernizing animal and other biomedical research facilities. The program aligns with the ORIP Strategic Plan 2021-2025 Strategy 2.2: Modernize the research infrastructure of laboratories and animal research facilities.
DCI program officials will hold a webinar for applicants to learn more about the program and obtain answers to questions that applicants may have. The webinar will be held on Friday, October 22, 2 pm-4 pm EDT. You may register here. Applications for PAR-21-326 are due on December 01, 2021.
We look forward to a productive fiscal year and continuing to fund research resources that support innovative biomedical research in partnership with other NIH Institutes, Centers, and Offices. To stay up-to-date with ORIP news and events, please follow us on Twitter and visit our website.
Franziska B. Grieder, DVM, Ph.D.
Director of the Office of Research Infrastructure Programs




