A New Method for Drosophila Cryopreservation Overcomes Longstanding Challenges
For biologists, cryopreservation holds major potential for numerous areas of research. The process of vitrifying (i.e., storing in glass to avoid the damaging effects of ice) biological samples—ranging from single cells to whole organisms—can be applied to such areas as biopharmaceutical testing, biological dressings, cell transplantation, and maintenance of genetic stocks.
Dr. John Bischof, a professor in the Department of Mechanical and Biomedical Engineering at the University of Minnesota (U of M), explained that the conceptually simple procedure of vitrifying biological samples reflects a complex optimization process to ensure viability. Dr. Thomas Hays, a U of M professor in the Department of Genetics, Cell Biology, and Development, cited the potential impact of the cryopreservation research effort in managing the large volume of Drosophila strains in laboratories, including national and international stock centers that are used by biomedical researchers around the world. Because live Drosophila stocks are costly and labor-intensive to maintain, cryopreservation would provide investigators with new capabilities for research.
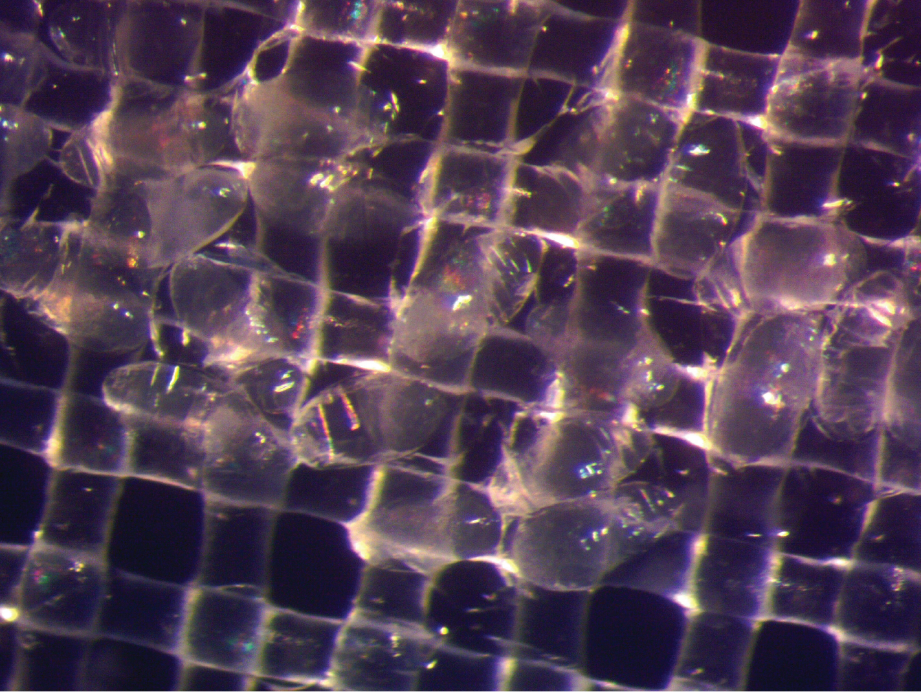
Drs. Bischof and Hays recently published a paper describing a simple, optimized approach for the cryopreservation of Drosophila melanogaster embryos and long-term storage in liquid nitrogen (Figure 1). The study was led by research associates Drs. Li Zhan and Min-Gang Li. The team’s research, which was supported by ORIP (R21OD028758), has filled a longstanding knowledge gap within the community.
“Many people know how to take immortalized cells that they grow in the lab, put 10% DMSO on them, and just throw them in the freezer. Because [the process] works in these immortalized cells, people think that there are no more problems to solve in cryobiology,” Dr. Bischof explained. “But there are problems, and Drosophila was a big one. So, we were really happy to make this work for the community.”
Dr. Zhan, the paper’s lead author, explained that researchers have faced three major challenges for Drosophila cryopreservation. First, the embryo’s vitelline membrane renders it impermeable to external chemicals. The membrane must be made permeable to allow the entry of cryoprotective agents (CPAs) into cells, which prevents ice crystallization and the resulting damage that otherwise would occur during the cryopreservation process.
The second challenge relates to timing for cryopreservation. The process must be performed at an optimal developmental stage. At early stages, the embryo is prohibitively fragile; at later stages, a cuticle layer forms, preventing permeabilization and uptake of CPAs. The third challenge relates to the embryo’s genetic background. Certain strains appear to tolerate the cryopreservation process better than others.
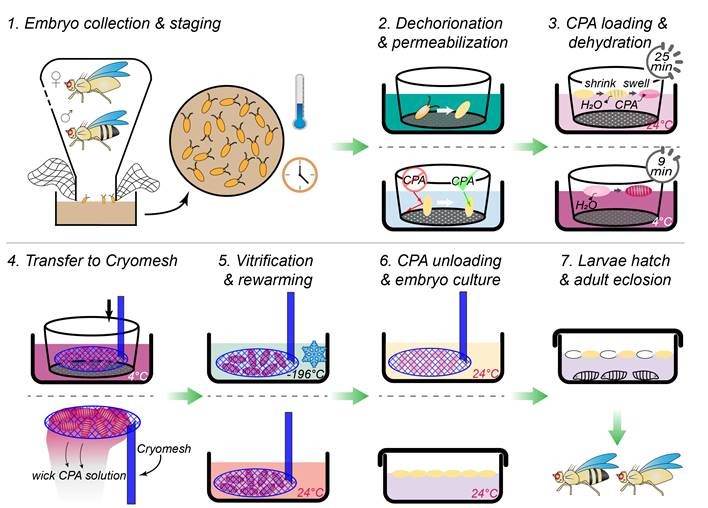
The team addressed these challenges in their protocol (Figure 2). They tested the process across multiple developmental stages, identifying the stage that yielded the best results. They also tested multiple CPAs and concentrations, identifying the combination that would minimize the effects of toxicity. Dr. Zhan emphasized that although other cryopreservation methods for Drosophila have been published, the team’s paper reports a simple yet robust approach for potential wide adoption by other laboratories.
“When we first started this project, we realized that there were two publications back in the 1990s that reported that they were able to get some survival of the [Drosophila] embryos,” Dr. Zhan explained. “But the device—the setup they used—was too complicated for other [fly] labs to reproduce. So, basically, we started there. We said, ‘Well, if we really want to work on the problem and make it work, that means we have to generate a simple enough approach so every other fly lab will be able to reproduce our results.’”
The team began by using simple devices and approaches. They trained non-specialists, including high school and other students who had no prior training in Drosophila or cryopreservation. These trainees were able to follow the protocol and yield favorable results. Dr. Zhan stated that presently the team is focused on training more investigators; the team is in contact with other research groups that are interested in learning the protocol.
ORIP has long been committed to addressing challenges for Drosophila cryopreservation within the biomedical research community. In 2016, ORIP and the National Institute of Neurological Disorders and Stroke co-sponsored the Cryopreservation of Drosophila Strains Workshop to evaluate the potential and practicality of developing efficient preservation methods for long-term storage of Drosophila stocks.
Dr. Li emphasized that development of reproducible, simple protocols for cryopreservation of Drosophila and other insects will advance biomedical and agricultural research. He highlighted, for example, the potential importance of the team’s findings for the implementation of biological control strategies to manage Drosophila suzukii (the spotted wing fruit fly), a major crop pest that infests and impacts yield of ripening soft fruits.
“We consider our method very accessible,” Dr. Li stated. “If you have a protocol that’s too complicated, no one will use it. Our protocol is practical, cheap, and very simple. We hope that after some limited training, people will be able to easily adopt the method.”
More information on ORIP’s efforts related to cryopreservation is available at orip.nih.gov/sites/default/files/ORIP-Cryopreservation-Fact-Sheet.pdf.
Reference
1 Zhan L, et al. Cryopreservation method for Drosophila melanogaster embryos. Nat Commun. 2021;12: 2412. doi:10.1038/s41467-021-22694-z.



