California National Primate Research Center Team Develops Novel Tau Model for Alzheimer's Disease in a Nonhuman Primate
Alzheimer’s disease (AD) affects more than 5.5 million Americans per year. The earliest symptoms of AD, however, often occur after decades of undetectable damage to the intricate synaptic connections within the brain. Many mechanisms of the disease’s progression remain a mystery for researchers, and effective therapeutics for AD have not been developed yet.
Dr. John Morrison, Director of the California National Primate Research Center (CNPRC) and a professor at the University of California, Davis, explained that AD’s slow progression reflects the remarkable resilience of brain circuits to damage. The neurodegeneration associated with AD appears to be related to the long-term accumulation of two proteins: tau, which aggregates to form neurofibrillary tangles within a neuron (Figure 1), and beta amyloid, which results in the formation of large plaques between neurons.
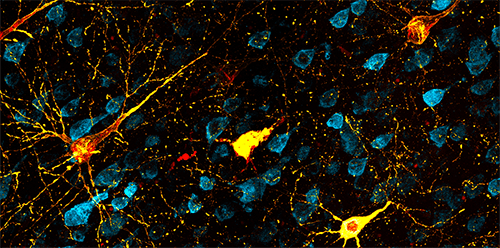
“This decade of pathology progression highlights the resilience of the brain,” Dr. Morrison emphasized. “Neural circuits can do pretty well when they lose 25% of neurons, but there’s a point at which you see collapse. You get to a point where it can’t function anymore, where too much of the circuit is damaged.”
Dr. Morrison’s team is developing nonhuman primate (NHP) models for AD that recapitulate the physiological effects of neurodegeneration associated with the disease. In an article published recently in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association,1 the team reported the development of the first tau model for early AD in rhesus macaques. This project was funded through supplemental funds to the parent award (P51OD011107) that represent a collaborative effort between the Office of Research Infrastructure Programs (ORIP) and the National Institute on Aging (NIA).
Dr. Manuel Moro, NIA Health Scientist Administrator, underscored the value of ORIP’s support for animal models in research on diseases of aging. He emphasized that the supplemental funds represent the value of collaborative efforts among NIH Institutes, Centers, and Offices. “The NIH and NIA are investing a lot of resources into [Alzheimer’s research],” Dr. Moro stated. “We don’t have all of the puzzle [pieces] together, but this paper shows that we can have a better model to try potential treatments.”
Rhesus macaques offer many advantages for the field of AD research because NHPs share a close genetic similarity with humans. Dr. Morrison reflected that previous studies in other model organisms—such as mice, worms, and fruit flies—have yielded powerful insights into specific biological pathways associated with AD. These models, however, often do not provide a complete outlook of AD progression in humans. In contrast, NHP models may yield new therapeutics by providing a closer biological link between the laboratory and clinic.
“We often say that we find the cure to cancer and Alzheimer’s disease in rodent models, but when we move to the clinic, it’s not working,” said Dr. Danielle Beckman, a postdoctoral fellow at CNPRC and the lead author of the Alzheimer’s and Dementia study. She explained that the team is not advocating for the replacement of mouse models but is developing an intermediate step for translational research. “If we can test therapies that work in mouse models prior to investing millions or billions of dollars into clinical trials, we really think it’s going to make an impact in having a new drug on the market. I think we really need to be open about new animal models for diseases.”
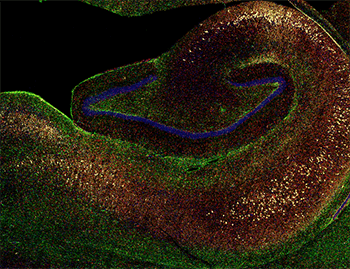
The team’s model took advantage of this genetic similarity, utilizing tau proteins that share a close homology between humans and macaques. In the early progression of AD, tau proteins are pathologically phosphorylated and bind to one another, leading to accumulation of the protein in fibrils within the brain. Typically, accumulation begins in the entorhinal cortex, which is involved in memory, and spreads to other brain regions. To induce this process in rhesus macaques, the team injected the animals with a double-mutant tau that contained two mutations associated with tau-based neuron death in humans (Figure 2).
“That’s kind of the Holy Grail,” Dr. Morrison emphasized. “We wanted to see if we could see neurons die along these pathways that are affected in humans and lead to cognitive decline.” He highlighted the work of his collaborators—including Dr. Jeff Kordower, a professor at Rush University Medical Center who recently moved to Arizona State University, and Dr. Paramita Chakrabarty, an assistant professor at the University of Florida—for their contributions to the project. Dr. Kordower is the team’s head surgeon and was involved in the microscopic analysis as well, and Dr. Chakrabarty has developed similar models in mice and provided the team a viral vector for injection of the mutant tau protein.
The team monitored signs of neuron death by collecting plasma and cerebrospinal fluid (CSF) samples. They also performed positron emission tomography imaging, which routinely is used for AD diagnostics. The effects of neurodegeneration were observed rapidly; within 3 months, end-stage tangles were present. The team now understands that within 6 months, the progress of neurodegeneration increases markedly. They also observed a neuroinflammatory response, suggesting that microglia might play a role in pathologic progression associated with the disease.
Drs. Morrison and Beckman emphasized that, although these results are promising, their work is far from complete. The team presently is seeking funding for several follow-up studies. In collaboration with Dr. Mark Baxter, a professor at the Icahn School of Medicine at Mount Sinai, Drs. Morrison and Beckman are planning behavioral studies in the model to detect subtle changes in memory and cognition. The team also is seeking industry funding for testing potential tau-based therapeutics.
The next step will be to characterize behavioral changes in the model. The team is using approaches that initially were developed by Dr. Baxter for other studies for the tau model of AD. Macaques are trained to perform computer‑based tasks that mirror the Wisconsin Card Sorting Test, a neuropsychological assessment often used in humans. Using this test, the team can detect subtle changes in performance. With these data, researchers can distinguish between key cognitive domains and gain insight into changes in the functions of the hippocampal and prefrontal regions over time. Paired with biochemical and imaging data, these tests will provide a full picture of AD progression in the model.
Dr. Beckman reflected on the project’s wide scope, noting that the CNPRC has played a crucial role in the team’s success. The CNPRC is one of the seven National Primate Research Centers (NPRCs) that support biomedical research across the United States. Funded by ORIP, the NPRCs possess a large team of staff members with expertise in maintaining NHP colonies, including veterinary practitioners, veterinary technicians, and a surgical team. Other staff members offer their expertise in such specialized procedures as imaging, necropsy, behavioral testing, and positive reinforcement training.
“It’s incredible how much vast support we have,” Dr. Beckman stated. “I think about what we’re doing—neurosurgery, [magnetic resonance imaging], blood and CSF collection—we’re really trying to extract as much [information] as we can. We cannot do this without the support of the Centers. There are a lot of people here making this project work.”
More information on the team’s work is available at themorrisonlab.org.
Reference
1 Beckman D, Chakrabarty P, Ott S, et al. A novel tau-based rhesus monkey model of Alzheimer’s pathogenesis. Alzheimers Dement. 2021;1–13. doi:10.1002/alz.12318.



