Swine Models Advance a New Option for Heart Valve Transplants in Neonates
Today, doctors treating neonates with critical congenital heart defects have two options: transplanting donor organs or using alternative approaches, such as a new heart valve transplantation procedure. Healthy heart valves are essential for ensuring unidirectional (single direction) blood flow. When an existing valve is diseased and needs replacement, a valve made from artificial materials, animal tissues, or a preserved valve from a human donor (an allograft) is frequently used; however, this approach has a serious limitation in children. Implanted valves cannot grow or repair themselves as the child and the heart grow. Therefore, the valve transplantation process must be repeated over time, exposing the patient to multiple life-threatening surgeries.

Recently, research on heart valve replacement has benefited from a unique ORIP-funded resource, the National Swine Resource and Research Center (NSRRC). Established in 2003, the NSRRC has created a number of genetically modified pigs in response to investigator-initiated requests and made them available to the biomedical community for studying a broad range of human diseases. Located at the University of Missouri’s Animal Science Research Center, the NSRRC also provides reagents, information, and training related to using swine models in research. Domestic swine (Sus scrofa domesticus) are useful for research studies because they share much of the same anatomy, genetics, and physiology with humans. This similarity has enabled researchers to use swine to successfully create a model to study coronary heart disease and possible interventions to treat it, as well as to use other models, including the green fluorescent protein–transgenic (GFP-Tg) swine, to conduct proof-of-concept studies to support the feasibility of a partial heart transplantation procedure in neonates.
Dr. Taufiek K. Rajab, Assistant Professor at the University of Arkansas for Medical Sciences (UAMS) (Figure 1) and his colleagues used NSRRC’s swine resources to develop a new partial heart transplantation procedure for neonates born with heart defects, to overcome the limitations of conventional valve transplantation in these pediatric patients. The NSRRC provided the GFP-Tg and the wild-type swine that were used as donors and recipients, respectively,1,2 in the team’s proof-of-concept studies (Figure 2). As predicted, animals survived, and the hearts (and valves) grew and functioned normally.
Dr. Rajab emphasized that this research would not have been possible without the unique service provided by the NSRRC. The NSRRC is the only provider of genetically modified GFP-Tg swine. Genetically modified rodents are effective models for many clinical challenges and are easy to source, but they cannot be placed on the heart–lung (“bypass”) machine required in open-heart surgery research. Piglets, however, can be placed on the same bypass machines that doctors use when performing surgeries on pediatric patients. Standard echocardiography (echo) machines also can be used with piglets. Although versions of the bypass and echo machines could be developed for rodents, the cost would be prohibitive. The NSRRC provided the swine model that enabled Dr. Rajab and his team to use readily available clinical tools and data to develop and test a new complex, experimental operation procedure.

Dr. Rajab first conceived of partial heart transplantation for neonates in 2019–2020 while a clinical fellow at the Children’s Hospital Colorado. He and his colleagues had seen the limitations of conventional valve replacement firsthand. He recollected how babies treated at his clinic received non-growing dimensional valve implants, which did not provide the best solution. The main issue with pediatric heart valve replacements, he noted, is that a child’s heart is still growing. When heart valves are surgically implanted in adults, they will function as intended for some time without needing to be replaced, and the outcomes are excellent. But in neonates, heart valve implants must be replaced repeatedly as the baby grows, and these surgeries have a high mortality rate. Dr. Rajab then came up with a new concept—to transplant not just the valve, and not the whole heart, but the part of the heart that includes the valves. He speculated that, as with a full heart transplant, the valves in a partial heart transplant would grow with the baby and spare the baby from undergoing multiple operations. Dr. Rajab also reasoned that partial heart transplants could reduce the risk of postsurgical complications. With full heart transplants, the pumping chambers of the heart begin to fail over time, leading to rejection. By transplanting only part of the organ, less tissue is at risk of the complications of transplantation. Valves also are more resistant than the pumping chambers to these complications. Dr. Rajab hypothesized that if these partial hearts could continue to grow successfully after transplantation, then this new surgical approach could be a viable option for growing heart valve replacements in neonates and children with heart valve defects.
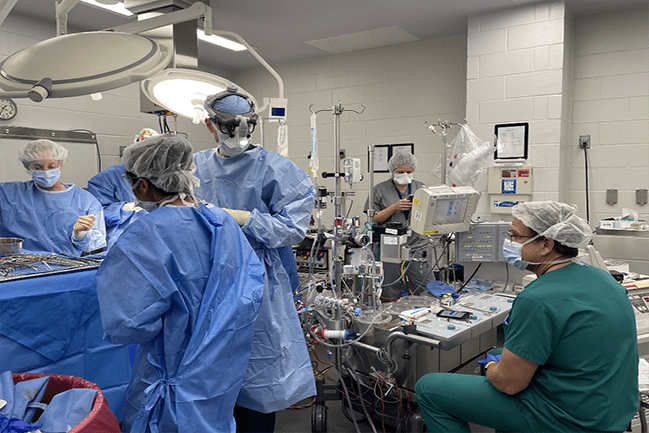
Dr. Rajab connected with NSRRC’s Dr. Randall Prather, Curators’ Distinguished Professor Emeritus, Division of Animal Sciences, University of Missouri, who helped Dr. Rajab receive immediate assistance with the swine model. Dr. Rajab then used this model with his institution’s clinical machines, including the bypass machine, the echo machine, and the anesthesia machine. The expertise of a clinical anesthesiologist and a clinical echocardiographer also was incorporated in the research studies (Figure 3). Describing the multidisciplinary team involved in the work, Dr. Rajab noted: “These are extremely highly trained health professionals who routinely perform these clinical procedures in babies.” The participation of highly trained pediatric surgeons in these studies is important because it helps to ensure that the procedure, once tested with piglets, can be rapidly translated into practice. The team was able to successfully perform these open-heart operations, for which having the swine model was key. Dr. Rajab reiterated: “The main point is that this would not have been possible without the NSRRC because no commercial vendor has these animals.”
Dr. Rajab has since collaborated with Dr. Joseph W. Turek, Associate Professor of Surgery and Associate Professor in Pediatrics at Duke University School of Medicine, to perform further partial heart transplantation experiments with swine piglets. After they confirmed their hypothesis, Dr. Turek’s team performed these surgeries in human babies, translating these findings to the clinic. In 2022, the team performed the first human partial heart transplant on a baby prenatally diagnosed with persistent truncus arteriosus and irreparable truncal valve dysfunction.3,4 This operation represented the transition of these research findings from bench to bedside. The first patient is now thriving with growing heart valves and is predicted to have the transplanted valves for life. Dr. Rajab remarked that this singular achievement “closes the circle that successful proof-of-concept studies in piglets enabled this surgery in human babies.”
Following the success of the initial operation, additional partial heart transplants have been successfully performed at Dell Children’s Medical Center, Duke Children’s Hospital, NewYork-Presbyterian Morgan Stanley Children’s Hospital, and UAMS. Dr. David M. Kalfa, Director of the Pediatric Heart Valve Center and Surgical Director of the Initiative for Pediatric Cardiac Innovation at Columbia University, is one of an integrated team of cardiologists and cardiothoracic surgeons at NewYork-Presbyterian and Columbia who have further advanced the procedure pioneered by Dr. Rajab by enabling two partial heart transplants to be performed using a single donor heart. This approach is known as the “domino” (i.e., sequential) partial heart transplant procedure. In a domino transplant, a donor heart is transplanted to a recipient needing a whole heart; then the valves from the heart being removed from the recipient are used for a partial heart transplant. The ability to help save more children with a single donated organ is a major step forward for doctors working with a limited number of pediatric donor hearts.
When considering future directions for research, Dr. Rajab observes that the next step is to better understand the transplant biology specific to partial heart transplant. Questions remain about the level of immune suppression needed, the time necessary for ischemia, and the differences in immunobiology of heart transplants. Partial heart transplants, for example, may require lower levels of immune suppression than full heart transplants because less tissue is being implanted. These questions can be explored through additional experimental open-heart operations conducted with the GFP-Tg swine model or through analyses using donor and recipient cells. He shared that the knowledge gained through continued research could be used to optimize treatment for children who receive these new partial heart transplants. “These are very complex experiments,” Dr. Rajab notes. “Without NIH support, we would not have been able to do this research in the piglets, which resulted in a new procedure that spares children who require heart valve substitutes from repeated operations as they grow.”
The NSRRC is a unique ORIP-supported resource. For more information, please visit ORIP’s Research Resources Directory webpage.
References
1 Bishara K, Kwon JH, Hill MA, et al. Characterization of green fluorescent protein in heart valves of a transgenic swine model for partial heart transplant research. J Cardiovasc Dev Dis. 2023;10(6):254. doi:10.3390/jcdd10060254.
2 Kang L, Aykut B, Javed H, et al. Partial heart transplant growth and hemodynamic function in piglets. J Am Coll Cardiol Basic Trans Science. 2025;10(4):502–504. doi:10.1016/j.jacbts.2024.10.015.
3 Turek JW, Kang L, Overbey DM, et al. Partial heart transplant in a neonate with irreparable truncal valve dysfunction. JAMA. 2024;331(1):60–64. doi:10.1001/jama.2023.23823.
4 Rajab TK, Ochoa B, Zilinskas K, et al. Partial heart transplantation for pediatric heart valve dysfunction: a clinical trial protocol. PLoS One. 2023;18(2):e0280163. doi:10.1371/journal.pone.0280163.



