Seeing Sound: UCSD Researchers Study Biology in a Whole New Light
Jesse V. Jokerst, Ph.D.
Associate Professor, Department of NanoEngineering
Affiliate Faculty, Materials Science Program
Adjunct Faculty, Department of Radiology
University of California, San Diego
When most people think of ultrasound, their first thought might be monitoring a fetus in utero. However, ultrasound has many applications in medicine beyond obstetrics, including oncology, cardiology, and orthopedics. Ultrasound imaging is perhaps the most widespread imaging modality, with a wide range of device sizes and applications. Ultrasound is a fast, accurate, and affordable method to diagnose disease and monitor a disease’s response to treatment: There are handheld scanners for remote clinics and research prototypes for advanced therapeutic monitoring applications. One of the biggest limitations to even broader use of ultrasound, however, is low contrast—it can be difficult to identify the specific cells, proteins, or anatomic structures of interest in the image.
What Is Photoacoustic Imaging?
One tool to increase the contrast of specific features in an image is called photoacoustic imaging. The photoacoustic effect was first described by Alexander Graham Bell in 1880 but has been used for medical imaging only in the last 20 years.
The photoacoustic effect generates sound via light. Conventional ultrasound imaging is “sound in/sound out”: The contrast is generated by differences in the reflection of the incident acoustic pressure waves off structures in the body. Photoacoustic ultrasound is “light in/sound out.” When the target tissue absorbs light, there is a spatially confined thermal expansion and thus no bulk heating of the tissue. This rapid thermal expansion creates a pressure difference that can be detected acoustically. That is, the light causes the tissue itself to generate sound rather than bouncing sound off of the tissue.
Photoacoustics has several advantages over both purely optical and purely acoustic approaches. First, it can dramatically increase the contrast of the images. This is because differences in light absorption between tissue types often are larger than differences in acoustic impedance between tissue types. Second, it is easier to see multiple channels of data. Traditional ultrasound largely is monochromatic. Because photoacoustics can use different wavelengths to excite the tissue, it can generate ultrasound in different color channels and thus offer greater insight into biological processes. Third, photoacoustics has a much higher resolution than optical techniques for deep tissue imaging because acoustic waves are not scattered by tissue nearly as much as light. Finally, photoacoustics can image both endogenous and exogenous targets: Hemoglobin, deoxyhemoglobin, and melanin all are excellent absorbers and can report tissue oxygenation. Alternatively, chemists can synthesize contrast agents that report a specific biological process via the photoacoustic signal.
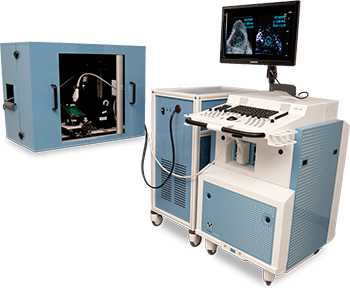
Photoacoustic imaging equipment can have various designs. One of the more popular designs is the VisualSonics Vevo LAZR (Figure 1). This system combines photoacoustic capabilities with high-frequency imaging. In both ultrasound and photoacoustics, the spatial resolution is a function of the frequency used for imaging: This resolution increases as the frequency increases but with a loss of penetration depth. Most hospitals and clinics use 5–12 MHz. The Vevo LAZR system offers a range of transducers from 12–70 MHz to facilitate a range of custom applications in both ultrasound and photoacoustics.
A team at the University of California, San Diego (UCSD), led by Dr. David Hall, installed a Vevo LAZR system in 2016 with support from the National Institutes of Health’s (NIH) Office of Research Infrastructure Programs’ (ORIP) S10 mechanism. Although housed in Moores Cancer Center on the UCSD campus, this tool has been used for several other applications beyond cancer imaging.
Dr. Jesse Jokerst’s group at UCSD is using photoacoustic imaging for acoustics-based stem cell imaging. Stem cell therapy is a powerful tool to treat disease, but a number of questions can arise when the cells are administered: How many cells are there? Where are the cells located? Are they alive or dead? Are they interacting with the diseased tissue? These are all questions that imaging is ideally suited to answer.
Magnetic resonance imaging is perhaps the gold standard in this field, but it is very expensive and has a long temporal resolution (i.e., it is like taking a picture). In contrast, acoustic imaging is incredibly fast and is like making a movie. This fast temporal resolution is important because it allows real-time guidance of the cell implantation event to ensure that a sufficient number of cells are administered to the target tissue.
Dr. Jokerst’s group has created nanoparticle contrast agents that allow physicians to image cells in real time.1–3 This group also invented nanoparticle contrast agents with multimodality imaging capabilities or combined therapeutic and diagnostic tools into nanoparticles to improve stem cell viability.4 As part of this focus on nanoparticle-based contrast agents, ORIP recently also has supported next-generation nanoparticle tracking analysis tools for UCSD via the S10 program (MANTA ViewSizer). The MANTA ViewSizer is a very accurate tool to measure the size, charge, and monodispersity of nanoparticle contrast agents—these are critical steps to clinical translation.
Photoacoustics for Use in Dental Imaging
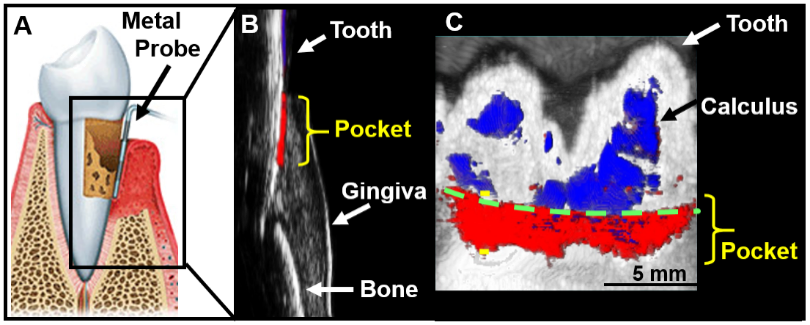
UCSD also has a very active research program in dental and periodontal photoacoustic imaging to characterize the gingiva and gingival disorders. The immediate goal is an improved approach to taking periodontal pocket depth measurements. This depth measurement is conventionally done with a metallic probe (Figure 2A), but this method gives highly variable values and is painful to the patient and time-consuming for the provider. Thus, Dr. Jokerst and colleagues developed an approach to measuring this non-invasively using photoacoustics (Figure 2B) in both swine models5 and healthy human subjects.6 The approach uses ink from cuttlefish (which also is used to dye pasta), because it is full of nanoparticles that have a strong photoacoustic signal. Dr. Jokerst’s vision is for dentists to be able to give patients an oral rinse and measure periodontal pocket depth using ultrasound machinery. The next part of this research is being conducted at the Herman Ostrow School of Dentistry at the University of Southern California—because UCSD does not have a dental school—in human subjects whose teeth and gums are not healthy.
Additionally, Dr. Jokerst’s laboratory soon will begin work on another dental health project: building a molecule that produces an ultrasound signal when dangerous bacteria are present in the mouth. This method will identify periodontal disease more easily, earlier, and more accurately than current tools do and will give dentists molecular-level insight into the disease to help better direct care and improve a patient’s quality of life.
Monitoring Heparin Anticoagulation Therapy
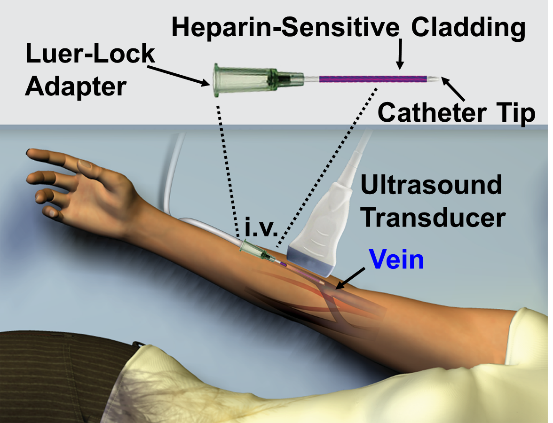
Another diverse application is monitoring heparin anticoagulation therapy. Heparin is a common anticoagulant but is very difficult to manage—particularly in high-dose scenarios such as in the operating suite or during extracorporeal membrane oxygenation. Dr. Jokerst’s group is supported by an NIH New Innovator Award to build an intravenous catheter that will be used to deliver and monitor heparin7 (Figure 3). This device has a heparin-sensitive cladding that produces an increased photoacoustic signal in response to heparin. A prototype of this device was used recently to study a cohort of human samples with good correlation to gold standard monitoring methods that require invasive blood samples.8
Improving Wound Care
Dr. Jokerst’s team also has used the ORIP-funded Vevo LAZR scanner to begin developing tools to improve wound care, including nanoparticles for potential use in imaging and treatment of bacterial infections.9,10 Other work on wound care has included research on imaging wounds. Using a mouse model, the researchers showed that the scanner’s imaging data could be used to determine the stage of an ulcer and even to detect an ulcer before it is visible by eye.11 After acquiring follow-up NIH funding, the team began a human-subject study, collecting data on such chronic wounds as foot ulcers suffered by people with diabetes and pressure ulcers experienced by residents of long-term care facilities. The results so far are encouraging, and the group is working to develop a 3‑dimensional map quantifying the extent of bone involvement and tissue oxygenation. The ability to detect such information before it can be seen by eye could be useful in the context of initiating treatment for such wounds. The imaging information also has the potential to be used to direct treatment and evaluate response to treatment.
What’s Next for Photoacoustic Imaging?
Dr. Jokerst and his collaborators have been working on other projects as well, including the use of nanoparticles for drug delivery. Many research groups have been able to image nanoparticle carriers successfully to obtain such information as their location and number, but it has been a challenge to determine whether the drug has been released from the carrier. Without drug release, the drug cannot treat the tumor or other target in the body. Dr. Jokerst’s team customized a standard chemotherapeutic drug inside nanoparticles so that it produces a photoacoustic signal when it is released.12 The researchers then are able to measure the signal to determine how much of the drug has been released.
Another effort in the photoacoustics space has involved imaging of the retina. One study, conducted in a rabbit model, demonstrated an amazing capability of the Vevo LAZR instrument—namely, quantifying the oxygenation of tissue, particularly in the retina. This capability has the potential to be highly useful for applications in ocular disease.13
All of these studies would be impossible without support from ORIP for the Vevo LAZR scanner. Although the photoacoustic imaging field is shifting away from the use of lasers—which can be complex and expensive—toward the more rugged, cheaper, and user-friendly light-emitting diodes (LEDs), the Vevo LAZR scanner will continue to remain a workhorse at UCSD (Figure 4).

References
1 Kim T, Lemaster JE, Chen F, Li J, Jokerst JV. Photoacoustic imaging of human mesenchymal stem cells labeled with Prussian blue-poly(l-lysine) nanocomplexes. ACS Nano. 2017;11(9):9022–9032.
2 Lemaster JE, Wang Z, Hariri A, Chen F, Hu Z, Huang Y, Barback CV, Cochran R, Gianneschi NC, Jokerst JV. Gadolinium doping enhances the photoacoustic signal of synthetic melanin nanoparticles: a dual modality contrast agent for stem cell imaging. Chemistry of Materials. 2018;31:251–259.
3 Chen F, Ma M, Wang J, Wang F, Chern S-X, Zhao ER, Jhunjhunwala A, Darmadi S, Chen H, Jokerst JV. Exosome-like silica nanoparticles: a novel ultrasound contrast agent for stem cell imaging. Nanoscale. 2017;9:402–411.
4 Lemaster JE, Chen F, Kim T, Hariri A, Jokerst JV. Development of a trimodal contrast agent for acoustic and magnetic particle imaging of stem cells. ACS Applied Nano Materials. 2018;1:1321–1331.
5 Lin CY, Chen F, Hariri A, Chen CJ, Wilder-Smith P, Takesh T, Jokerst JV. Photoacoustic imaging for noninvasive periodontal probing depth measurements. Journal of Dental Research. 2018;97:23–30.
6 Moore C, Bai Y, Hariri A, Sanchez JB, Lin C-Y, Koka S, Sedghizadeh P, Chen C, Jokerst JV. Photoacoustic imaging for monitoring periodontal health: A first human study. Photoacoustics. 2018;12:67–64.
7 Wang J, Chen F, Arconada-Alvarez SJ, Hartanto J, Yap L-P, Park R, Wang F, Vorobyova I, Dagliyan G, Conti PS. A nanoscale tool for photoacoustic-based measurements of clotting time and therapeutic drug monitoring of heparin. Nano Letters. 2016;16:6265–6271.
8 Jeevarathinam AS, Pai N, Huang K, Hariri A, Wang J, Bai Y, Wang L, Hancock T, Keys S, Penny W. A cellulose-based photoacoustic sensor to measure heparin concentration and activity in human blood samples. Biosensors and Bioelectronics. 2019;126:831–837.
9 Kim T, Zhang Q, Li J, Zhang L, Jokerst JV. A gold/silver hybrid nanoparticle for treatment and photoacoustic imaging of bacterial infection. ACS Nano. 2018;12:5615–5625.
10 Mantri Y, Davidi B, Lemaster JE, Hariri A, Jokerst JV. Iodide-doped precious metal nanoparticles: Measuring oxidative stress in vivo via photoacoustic imaging. Nanoscale. 2020;12:10511–10520.
11 Hariri A, Chen F, Moore C, Jokerst JV. Non-invasive staging of pressure ulcers using photoacoustic imaging. Wound Repair and Regeneration. 2019;27:488–496.
12 Jeevarathinam AS, Lemaster JE, Chen F, Zhao E, Jokerst JV. Photoacoustic imaging quantifies drug release from nanocarriers via redox chemistry of dye-labelled cargo. Angewandte Chemie International Edition. 2020;59:4678–83.
13 Hariri A, Wang J, Kim Y, Jhunjhunwala A, Chao DL, Jokerst JV. In vivo photoacoustic imaging of chorioretinal oxygen gradients. Journal of Biomedical Optics. 2018;23(3):1–8.



