Promising One-Dose Rapid Treatment for Newborns to Prevent Lifelong Infection with HIV
Newborns can be exposed to HIV—the virus that causes AIDS—during gestation, birth, or breastfeeding. In general, babies born to mothers who test positive for HIV are screened and tested. If found positive, the babies will receive the standard of care: antiretroviral therapy (ART) treatment. Because no cure currently exists for HIV, these babies will receive treatments for the rest of their lives to keep the virus suppressed (i.e.,below detectable levels).
In the United States, vertical transmission from mothers with HIV to their infants is exceedingly rare. However, HIV infection in newborns remains a problem internationally. According to the Joint U.N. Programme on HIV/AIDS 2018 report, an estimated 180,000 new HIV infections occurred in children younger than 15 years of age in 2017, and most of these infections were acquired during or shortly after birth.1 Transmission during birth is drastically reduced by treating the mother with ART before the baby’s birth (natural or cesarean section), as well as by the use of formula feeding to prevent transmission through breastmilk. Access to ART to treat mothers before delivery and newborns with HIV is limited in low-resource areas, which presents a significant barrier to effectively treating these patients.
Researchers at the Oregon National Primate Research Center (ONPRC) are developing a short-term therapy to treat HIV infection in newborns born to mothers infected with the virus, providing an accessible, low-cost way to prevent lifelong HIV infection among these children. ONPRC Director Dr. Nancy L. Haigwood, her graduate student Mariya B. Shapiro, and ONPRC colleagues have optimized a simian-human immunodeficiency virus (SHIV) model in newborn rhesus monkeys treated with protective HIV antibodies that were provided by scientists at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center.
The data generated by the SHIV model developed at ONPRC suggest the potential for an HIV cure if treatment is started within the first 2 days after birth. Studies using this model looked at a combination therapy consisting of two broadly neutralizing antibodies (bNAbs): PGT121 and VRC07-523.2 These studies found that a single-dose antibody cocktail given 30 hours after oral high-dose exposure to SHIV fully protected the newborn animals. Only 50 percent of the animals treated after 48 hours with repeated lower doses of the same antibody cocktail were free of the virus. These studies also suggested that treating the animals with only ART for just 3 weeks was sufficient to provide sustained protection against HIV if the animals had no additional exposure to the virus (Figure 1). Further investigations are needed to validate these preclinical findings in a clinical setting.
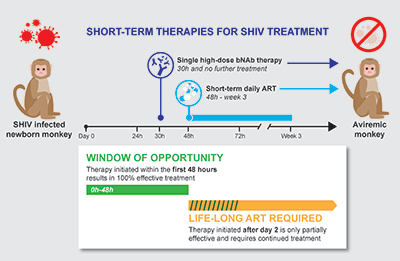
The high-dose, single-antibody cocktail approach seems to be remarkably effective—as successful as ART—and is superior to multiple-dose exposures (tested in prior studies3). Because of the different mechanisms of action, Dr. Haigwood pointed out combining ART with bNAbs has the potential to become an HIV cure and will positively affect HIV research in the years to come. “It is an exciting time for the ONPRC and HIV researchers, such that tractable, single short-term treatments that can fully clear the infection/virus for babies born to mothers testing positive for HIV are becoming available,” said Dr. Haigwood.
The ONPRC, one of seven Centers comprising the National Primate Research Centers Consortium, is funded, in part, by a grant through the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health (NIH). The ONPRC investigators have shared their primate research findings broadly across the Consortium and the scientific community, including in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network.
Clinical trials evaluating human monoclonal antibodies (mAb) effective against HIV, including one of those (VRC07-523LS) that Dr. Haigwood and colleagues used in optimizing the infant SHIV model, are ongoing in the IMPAACT Network. In one such vertical transmission trial, investigators have started to assess a combination of ART with VRC01, which is the only bNAb approved by the U.S. Food and Drug Administration for HIV treatment in newborns.4 Trial investigators are starting to administer VRC01 to patients at earlier time points, and results will be made available as the study progresses.
Recognizing that clinical trials routinely are done with an individual agent, the next step Dr. Haigwood proposes for vertical transmission studies will be to evaluate antibody cocktails in humans, particularly in babies who were exposed to HIV during birth or by breastfeeding, and also in combination with ART. “Generally, in the field, use of antibody therapy as prophylaxis employs the use of more than one monoclonal antibody to ensure full protection against the virus,” explained Dr. Haigwood.
The potential to directly translate the results of studies using the SHIV model to a clinical trial demonstrates the utility of the NHP model. As clinical trials on mAb treatments progress, the promise of an HIV cure for children born to infected mothers will become increasingly likely.
References
1 Joint U.N. Programme on HIV/AIDS. Miles to Go: Closing Gaps, Breaking Barriers, Righting Injustices. Geneva: United Nations:89. Available at: https://reliefweb.int/sites/reliefweb.int/files/resources/miles-to-go_en.pdf. Accessed October 15, 2020.
2 Shapiro MB, Cheever T, Malherbe DC, et al. Single-dose bNAb cocktail or abbreviated ART post-exposure regimens achieve tight SHIV control without adaptive immunity. Nat Commun. 2020;11:70.
3 Hessell AJ, Jaworski JP, Epson E, et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med. 2016;22:362–368.
4 Cunningham CK, McFarland EJ, Morrison RL, et al. Safety, tolerability, and pharmacokinetics of the broadly neutralizing human immunodeficiency virus (HIV)-1 monoclonal antibody VRC01 in HIV-exposed newborn infants. J Infect Dis. 2020;222:628–636.



