ORIP Funds Research to Advance Large Animal Models Using Stem Cell Transplantation
Many biomedical researchers use animal models to better understand diseases that occur in humans. Transplantation of testis stem cells—the foundation of sperm production and male fertility—is an established approach developed in mice, but its application to large animals (e.g., sheep, pigs, nonhuman primates) has been more challenging. Dr. Ina Dobrinski, a professor at the University of Calgary, has received funding from ORIP (R01OD016575) to explore this topic.
Dr. Dobrinski is developing new technologies for germline stem cell–based gene editing to generate pig models for human disease (Figure 1). Compared to mice, pigs are more closely related to humans in their biology and offer the potential to help researchers advance new treatments for diseases. Pig models have been developed for various diseases, including cystic fibrosis, diabetes, and neurofibromatosis type I. Dr. Dobrinski’s research aims are to (1) establish a cell-culture system that promotes pig germline stem cell expansion in vitro, (2) explore the role of testicular somatic cells in formation of a functional niche, and (3) develop a precise approach for targeted gene editing in pig germline stem cells.
Over the past two decades, Dr. Dobrinski has played a key role in establishing germline stem cell transplantation and demonstrating transgene transmission in large animals, as well as achieving precise gene editing in large animal germline cells.1,2 Currently, her team is exploring and optimizing approaches to culture these cells in the laboratory, an effort that she referred to as “one of the biggest challenges to the technology of germline gene editing.” Dr. Dobrinski remarked that researchers have attempted to apply the previously established approaches in mice to other animals, but these efforts have often been unsuccessful to date. She underscored the need for more progress in this area.
“When we’re dealing with a human or pig testis, we’re dealing with different cells than those that come from a mouse testis. These cells have different metabolic requirements, different epigenetic stages,” Dr. Dobrinski explained. Epigenetics is a rapidly growing area of science that focuses on the processes that help direct when individual genes are turned on or off. “We all thought the mouse system would work [in large animals] with just a little bit of tweaking, but 25 years later it still really doesn’t. So, we have to do something fundamentally different. It needs a different approach.”
One of these potential approaches is xenotransplantation, a technique that involves transferring gene-edited cells or tissues from larger animals into a mouse. Dr. Dobrinski explained that these xenografts serve as intermediaries between in vitro models and the species of interest. The xenograft mice also can be maintained more readily in most laboratories than large animals, enhancing researchers’ capabilities to use these models for their studies.3
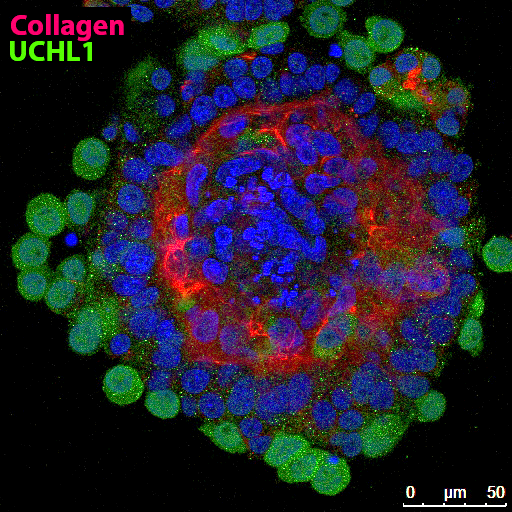
The team is also working to develop organoids (i.e., “mini-organs” composed of 3D tissue cultures) from pig testis cells (Figure 2). Dr. Dobrinski explained that organoids exist in an environment that more closely mimics their natural environment, compared to traditional cell culture dishes or suspensions. The organoid offers researchers the potential to study cellular processes and communications in a setting that is closer to in vivo conditions.4
Overall, Dr. Dobrinski underscored the importance of these experiments to advance the field of large animal model development for biomedical research, particularly in situations where access to human tissues is limited. “We need these translational models between mouse and humans, and the biomedical field wouldn’t have progressed the way it has without having them,” Dr. Dobrinski emphasized. “I am grateful to ORIP for recognizing the importance of this need and supporting this effort throughout my entire career.”
ORIP is committed to ensuring that scientists have access to large animal models and recognizes that these models provide unique translational opportunities for developing and testing novel devices, diagnostic tools, and therapies that can be used in humans. For more information on ORIP’s support for developing large animal models, please review ORIP’s fact sheet, and for additional information on this topic, please visit ORIP’s website.
References
1 Zeng W, Tang L, Bondareva A, et al. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biol Reprod 2013;88(1):27. doi:10.1095/biolreprod.112.104422.
2 Webster D, Bondareva A, Solin S, et al. Targeted gene editing in porcine spermatogonia. Front Genet 2021;11:627673. doi:10.3389/fgene.2020.627673.
3 Honaramooz A, Snedaker A, Boiani M, et al. Sperm from neonatal mammalian testes grafted in mice. Nature 2002;418(6899):778–781. doi:10.1038/nature00918.
4 Sakib S, Uchida A, Valenzuela-Leon P, et al. Formation of organotypic testicular organoids in microwell culture. Biol Reprod 2019;100(6):1648–1660. doi:10.1093/biolre/ioz053.



