ORIP-Funded Instrumentation Supports Structural Studies for Next-Generation Pan-Sarbecovirus Vaccines
In the past 20 years, two sarbecoviruses—SARS-CoV-1 and SARS-CoV-2—have caused major disease outbreaks in humans. Researchers are interested in developing pan-sarbecovirus vaccines with stronger cross-neutralizing protective effects to resist viral evolution and prevent future outbreaks. Through the S10 Instrumentation Grant Programs, ORIP is helping this effort by funding shared resources for structural and functional analyses of viral proteins to enable studies of vaccine effectiveness. Dr. David Veesler, Hans Neurath Endowed Chair in Biochemistry at the University of Washington (UW) and Investigator, Howard Hughes Medical Institute, underscored the importance of these studies. “We build on the fundamental knowledge we generate from the understanding of viral entry and immunity against these viruses to guide our translational program in developing antibody therapies and vaccines,” he explained.
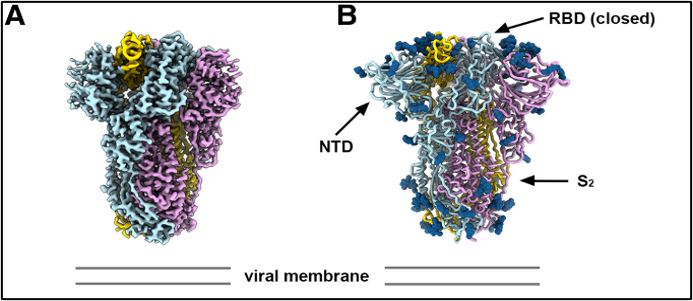
Sarbecoviruses use spike (S) proteins to recognize angiotensin-converting enzyme 2 (ACE2) receptors on host cell membranes. Dr. Veesler and his research team at UW were interested in characterizing the properties of S proteins expressed by other members of the sarbecovirus family. His group studied PRD-0038, a clade 3 sarbecovirus discovered in Africa (Figure 1). The team’s initial studies indicated that the PRD0038 S protein interacts strongly with ACE2 proteins from several host species and that certain mutations could promote binding of the PRD-0038 S protein to human ACE2. Dr. Veesler’s group used cryo-electron microscopy (cryo-EM) to gain insights into the receptor’s ability to infect selective cells and induce host immune response to the S protein.

Cryo-EM is a powerful technique for determining the 3D structure of biologically relevant molecular and macromolecular complexes. Cryo-EM involves imaging vitrified samples (i.e., samples in a frozen–hydrated state, in which water is solidified without ice crystallization) and then processing hundreds of thousands of sample images into a 3D structure model. Unlike other structural techniques, cryo-EM enables imaging of sample mixtures and whole cells. Recent technological advances in cryo-EM have enabled researchers to image structures at near-atomic resolution. In 2017, UW received funds from ORIP (S10OD023476) to acquire a high-performance cryo-electron microscope. The instrument, which is housed in the Arnold and Mabel Beckman Cryo-EM Center (Beckman Center) at UW (Figure 2), provides researchers with access to high-quality data from what has emerged as a leading structural biology technique.

Dr. Justin Kollman, the principal investigator on the S10 award, is the Director of the Beckman Center. He explained that when the facility was first established in 2017, ORIP funding was vital for solidifying support from philanthropic organizations, such as the Murdock Trust (Figure 3). “Once we got the NIH money, it really helped with some of the last pieces of equipment. We needed cameras for the microscopes, especially. That was critical. Amazingly, all of that money came together at the same time,” recalled Dr. Kollman. “Nobody wanted to commit until they knew that everybody had committed.” According to Dr. Kollman, the Beckman Center supports NIH-funded UW investigators and local users from other academic institutions and smaller biotechnology and pharmaceutical companies. “At least a dozen different UW labs routinely use the instrument now, working in a variety of different biomedical research fields,” Dr. Kollman stated.
The team characterized the structure of the PRD-0038 S protein in its prefusion (or unbound) form and examined the effects of mutations on the protein’s binding abilities. To identify potential candidate antibodies for pandemic preparedness, a panel of monoclonal antibodies that have shown broadly neutralizing activity was probed against the PRD-0038 S protein. The study authors suggest that including clade 3 S proteins in future vaccines will elicit broader immunogenicity and increase resistance to viral evolution.1 The study also utilized flow cytometry and high-performance computing resources supported by S10 awards from ORIP (S10OD026959 and S10OD021644, respectively). These instruments were used for the deep mutational scanning analysis carried out in the Starr Lab at The University of Utah. The group has received funding to continue the sarbecovirus project through a phase 1 clinical trial.
Dr. Veesler emphasized that funding from ORIP and NIH was essential for the project: “The S10 funding allowed us to acquire one of the two microscopes that we have in our cryo-EM center, which was instrumental for this work. It wouldn’t have been possible without all the federal funding we received from NIH.” Dr. Veesler also underscored the importance of this work for vaccine development. “Sarbecoviruses are so diverse that if you want to elicit neutralizing antibodies—and therefore decent protection against that phylogenetic diversity—you need to span the diversity in a vaccine candidate to effectively protect from more viral strains and viral evolution,” he remarked. “That’s the basis for the clinical advancement of a vaccine we are developing.”
ORIP’s S10 programs support the purchase of commercially available instruments to enhance research by NIH-funded investigators. Each S10-funded instrument must be shared among multiple users, which ensures efficient use of instruments for research operations, enhances the cost-effectiveness of NIH’s investment, and maximizes benefits for thousands of investigators in hundreds of institutions nationwide. “I think [this study] is a very good example of the types of work NIH funds that have the potential to change our lives with actual drugs and vaccines,” added Dr. Veesler. “The importance of supporting fundamental science is really, really key.”
For more information on the S10 programs, please visit the ORIP website.
Reference
1Lee J, Zepeda SK, Park Y-J, et al. Broad receptor tropism and immunogenicity of a clade 3 sarbecovirus. Cell Host & Microbe 2023;31(12):1961–1973.e11. doi:10.1016/j.chom.2023.10.018.



