New Visualization Method Gets to the Heart of Parkinson's Disease
Parkinson's disease (PD), the second most common progressive neurodegenerative disorder affecting U.S. adults over the age of 60, is predicted to increase in prevalence as the American population ages.1 The characteristic motor-related symptoms are rest tremor, rigidity, bradykinesia, and stooping posture. Lesser known is the loss of cardiac postganglionic sympathetic innervation, which is a characteristic pathology of PD that progresses over time, is independent of motor symptoms, and is not responsive to typical anti-Parkinsonian therapies. Dr. Marina Emborg is a senior scientist at the Wisconsin National Primate Research Center (WNPRC) and Professor of Medical Physics at the University of Wisconsin–Madison (UW–Madison). She has been working on preclinical models of neurodegenerative disorders and the development of novel therapeutic strategies since 1989 and has made it her main research goal to help find a cure for PD. She describes reports indicating that by the time PD patients are diagnosed, typically based on motor symptoms, about 60 percent also have serious damage to the nerve connections in the heart from the sympathetic nervous system.2 Dr. Emborg added: “When healthy, these nerves stimulate the heart to accelerate its pumping to rapidly respond to changes in activity and blood pressure. Loss of this control causes patients to be less responsive to exercise, subjected to intense lightheadedness upon standing, and experience a high risk of falling.”
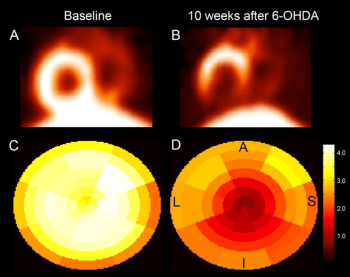
Dr. Emborg, Dr. Valerie Joers—who was then Dr. Emborg’s graduate student—and other UW–Madison colleagues, including cardiovascular medicine professor Dr. Timothy Kamp and neurology professor Dr. Catherine Gallagher, developed a new nonhuman primate (NHP) model in which they mimicked this PD cardiac sympathetic neurodegeneration in adult rhesus macaques using systemic dosing of the neurotoxin 6 hydroxydopamine (6-OHDA).3,4 These early studies included both sexes; however, no sex differences were evident so the group focused on male adults since the relative risk of developing PD is one and a half times higher in men than women. Following the establishment and characterization of this model by Dr. Joers, a second Emborg laboratory graduate student, Dr. Jeanette Metzger, along with colleagues at UW–Madison, used the model to follow nerves within the hearts of living animals using positron emission tomography (PET) imaging after they administered new-generation radiolabeled biomarkers (i.e., radioligands) to detect loss of heart nerves and related inflammation and oxidative stress (Figure 1).5
Remarkably, Dr. Metzger and her colleagues were successful in detecting inflammation and signs of oxidative stress in nerves as they were deteriorating (i.e., in real time). The study suggests that cardiac PET imaging combined with these new-generation radioligands will be useful in detecting heart disease and in evaluating new therapies that specifically target nerve disease within the human heart. Dr. Emborg emphasizes that patients are at different stages of the disease when they are seen by a clinician and that this NHP PD cardiac dysautonomia model allows the researcher the necessary control to study neurodegeneration as it is occurring. Dr. Metzger emphasized, as lead author of the study,5 “We know there is damage in the heart in Parkinson’s, but we haven’t been able to look at exactly what’s causing it. Now we can visualize in detail where inflammation and oxidative stress are happening in the heart, and how that relates to how Parkinson’s patients are losing those neuronal connections in the heart.6
“Many doctors are not aware of this condition, which significantly affects PD patients’ health,” said Dr. Emborg. After the study results had been published, several PD patients reached out to her to say, “Thank you for studying this aspect of the disease.” Dr. Emborg realized that this study gave patients the evidence and confidence they desired to have conversations with their doctors about treatments. Dr. Emborg has two main messages she would like to convey to the research community about PD and the heart: (1) The information being shared helps patients better communicate with their doctors and (2) Other diseases share this problem. In fact, diabetes, heart attacks, and other disorders cause similar damage to nerves in the heart, and these patients also could benefit from this new visualization model.7–9 Another particularly important aspect of having such a model is the ability to test potential therapies that could significantly improve patient outcomes.
Regarding the critical translational aspects of this research, Dr. Emborg envisions one day using this technique in a clinical setting to assist in developing new therapies for patients, as well as using this cardiac biomarker signature as a tool to predict high-risk PD patients. Her next steps are to refine and develop improved models of PD that reflect the non-motor-related symptoms.
Dr. Emborg has been engaged in NHP research at the WNPRC since 2004. She also heads the UW–Madison Preclinical Parkinson’s Research Program, under which her laboratory strives to identify neglected issues, discover gaps in knowledge, and collaborate with other groups to accelerate the process of translating findings into the clinic. Notably, the WNPRC is one of seven Centers comprising the National Primate Research Centers Consortium. It is funded in part by a grant through the Office of Research Infrastructure Programs of the National Institutes of Health. The development of the model described above was made possible by a 2010–2011 UW–Madison Clinical and Translational Science Award WNPRC Pilot Research Project award.
References
1 Beitz JM. Parkinson’s disease: A review. Frontiers in Bioscience (Scholar Edition). 2014;6:65–74.
2 Kashihara K, Imamura T, Shinya T. Cardiac 123I-MIBG Uptake is reduced more markedly in patients with REM sleep behavior disorder than in those with early-stage Parkinson’s disease. Parkinsonism & Related Disorders. 2010;16(4):252–255.
3 Joers V, Dilley K, Rahman S, et al. Cardiac sympathetic denervation in 6-OHDA-treated nonhuman primates. PLoS One. 2014;9(8):e104850.
4 Joers V, Seneczko K, Goecks NC, et al. Nonuniform cardiac denervation observed by 11C-meta-hydroxyephedrine PET in 6-OHDA-treated monkeys. PLoS One. 2012;7(4):e35371.
5 Metzger JM, Moore CF, Boettcher CA, et al. In Vivo Imaging of inflammation and oxidative stress in a nonhuman primate model of cardiac sympathetic neurodegeneration. NPJ Parkinson’s Disease. 2018;13;4:22.
6 School of Medicine and Public Health, University of Wisconsin–Madison. Researchers Trace Parkinson’s damage in the heart. Updated July 30, 2018. Accessed June 4, 2020. https://www.med.wisc.edu/news-and-events/2018/july/researchers-trace-parkinsons-damage-in-heart/
7 Miyamoto, T, Miyamoto M, Inoue Y, et al. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology. 2006;67(12);2236–2238.
8 Kashihara K, Ohno M, Kawada S, Okumura Y. Reduced cardiac uptake and enhanced washout of 123I-MIBG in pure autonomic failure occurs conjointly with Parkinson’s disease and dementia with lewy bodies. Journal of Nuclear Medicine. 2006;47;1099–1101.
9 Gerson, MC, Caldwell JH, Ananthasubramaniam, K, et al. Influence of diabetes mellitus on prognostic utility of imaging of myocardial sympathetic innervation in heart failure patients. Circulation: Cardiovascular Imaging. 2011;4(2):87–93.



