Immune Compromised Zebrafish: A Versatile and Cost-Effective Xenograft Model
Cell transplantation studies using immune compromised animals have advanced our understanding of the generation of immune cells, stem cell biology, cancer, and regenerative medicine. Over the past decade, a wide array of these immune compromised animal models, such as mice, rats, and pigs, have been developed. These animals have severe defects in the types and numbers of T, B, and natural killer (NK) cells as shown by a decrease or absence of these cell types. This allows for xenograft engraftment, transfer of cells or tissue from a wide array of animal species into the immune compromised animal.
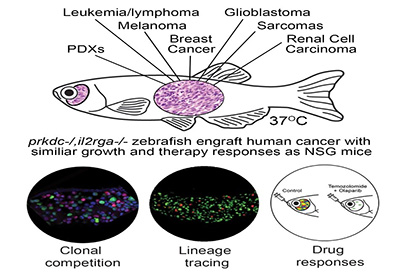
Despite the development of immune compromised mammalian models, there remains a need for the development of animal models that accurately reflect the human disease state but that are cost effective, permit large scale experimentation and screening, and allow facile imaging of transplanted cell processes at single cell resolution. A research group led by David Langenau, Ph.D., at Massachusetts General Hospital, in collaboration with a research team led by John Rawls, Ph.D., from Duke University, has developed numerous types of immune compromised zebrafish models that have varying degrees of immune deficiency. The zebrafish models were developed by creating changes in zebrafish genetic material and specialized zebrafish rearing techniques. Logistically, these small animals require minimal space to maintain, are low-cost, and produce numerous offspring. Most of these models can accept engrafted allogeneic tissue, within the same species tissue transfer; however, one model is able to accept xenografts or tissue from a human (Table 1). Several additional models, as yet, remain uncharacterized.
| Line | ZFIN ID | Phenotype | Publication (PMID #) | Status |
|---|---|---|---|---|
| rag2 E450fs (AB) | fb101 | T-, B-low | 25042784 | Available |
| rag2 E450fs (casper) | fb101 | T-, B-low | 26790525 | Available |
| jak3 P369fs (casper) | fb102 | T-, NK- | 27810924 | Available |
| prkdc D3612fs (casper) | fb103 | T-, B- | 27810924 | Available |
| il2rga Y91fs (casper) | fb104 | T-, NK- | 28878000 | Available |
| il2grb I30fs (casper) | Pending | Not Characterized | Pending | Available |
| zap70 Y442fs (casper) | y442 | T- | 27601584 | Available |
| prkdc D3612fs, il2rga Y91fs (casper) | Pending | T-, B-, NK- | Submitted | Pending |
| nk-lysin 1-4 knockout | Pending | Not Characterized | Pending | Pending |
| rag2 knockout (casper) | Pending | Not Characterized | Pending | Pending |
Table 1. Currently available zebrafish lines produced by the Office of Research Infrastructure Programs (ORIP), National Institutes of Health (NIH)-supported Immune Deficient Zebrafish Resource (R24OD016761). This table has been reprinted by permission of the author, Dr. David Langenau.
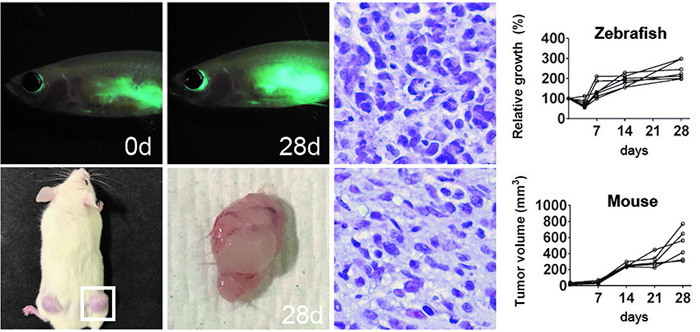
Before the development of these immune compromised models, transfer of allogeneic zebrafish tissue or xenogeneic tissue from a human donor for engraftment could only take place in adult zebrafish recipients that had their immune systems weakened by exposure to radiation. Long-term studies of engrafted tissue in these recipients were not feasible as the recipient fish immune systems would regain function and reject the engrafted tissue. Most of these models also have been combined with zebrafish, which have a genetic background that makes them transparent. This ensures an optically clear fish in which the growth and spread of cancer cells can be observed. Thus, optically clear immune compromised zebrafish are a potentially powerful model for studying the long-term growth and spread of cancer cells in an animal host. Fluorescent labeling of cancer cells can allow for observation of individual cancer cells in the immune compromised optically clear zebrafish as well.
An optically clear immune compromised zebrafish model developed by the Langenau laboratory accepts xenografted cells from species such as humans or mice. Previously, xenograft cell transplantation studies in zebrafish have been conducted in 2-day old zebrafish larvae prior to the development of the acquired immune system. When the immune system matured, the engrafted cells were rejected. A specialized, optically clear zebrafish model, that lacks T, B, and NK cells, was recently developed that accepted xenograft transfer of mouse and human cancer cells.
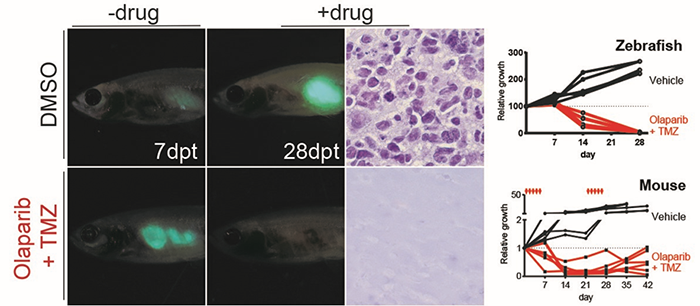
In addition, special rearing techniques have been developed to help achieve long-term survival and high engraftment rates for immune deficient fish engrafted with human cells and maintained at 37ºC. When combined with innovative techniques for oral administration of a drug into the stomach, it is also now possible to provide accurate drug dosing and schedules required for pre-clinical evaluation of cancer therapies.
The development of the immune compromised zebrafish models was supported by an R24 Resource Development grant from ORIP at the NIH. Approximately 5,000-10,000 adult zebrafish are maintained (housed) at any given time. Immune compromised zebrafish lines created by Dr. Langenau’s team are distributed to the biomedical community (https://www.langenaulab.com/fish-orders).
With even more advanced development of the immune compromised zebrafish models, these models will likely permit the robust engraftment of patient-derived cells/tissues, including embryonic stem cells, induced pluripotent cells, muscle and blood cells, and serve as a versatile animal model for the most optimal new drugs for a wide array of indications.


References
Tang Q, Abdelfattah NS, Blackburn JS, Moore JC, Martinez SA, Moore FE, Lobbardi R, Tenente IM, Ignatius MS, Berman JN, Liwski RS, Houvras Y, Langenau DM. Optimized cell transplantation using adult rag2 mutant zebrafish. Nature Methods. 2014;11(8):821-4.
Tenente IM, Tang Q, Moore JC, Langenau DM. Normal and malignant muscle cell transplantation into immune compromised adult zebrafish. Journal of Visualized Experiments. 2014;(94).
Tang Q, Moore JC, Ignatius MS, Tenente IM, Hayes MN, Garcia EG, Torres Yordán N, Bourque C, He S, Blackburn JS, Look AT, Houvras Y, Langenau DM. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nature Communications. 2016;7:10358
Moore JC, Tang Q, Yordán NT, Moore FE, Garcia EG, Lobbardi R, Ramakrishnan A, Marvin DL, Anselmo A, Sadreyev RI, Langenau DM. Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish. Journal of Experimental Medicine. 2016;213(12):2575-2589.
Yan C, Brunson DC, Tang Q, Do D, Iftimia NA, Moore JC, Hayes MN, Welker AM, Garcia EG, Dubash T, Hong X, Drapkin BJ, Myers DT, Phat S, Volorio A, Marvin DL, Ligorio M, Dershowitz L, McCarthy KM, Karabacak M, Fletcher JA, Sgroi D, Iafrate AJ, Maheswaran S, Dyson NJ, Haber DA, Rawls JF, Langenau DM. Visualizing human cancer engraftment and therapy responses at single-cell resolution using optically-clear immune deficient zebrafish. Cell. 2019 In press.



