Flow Cytometer at West Virginia University Supports Research Across Disciplines
A state-of-the-art flow cytometer awarded to West Virginia University (WVU) in 2013 has helped investigators make scientific advances across a remarkably wide range of disciplines. West Virginia is among the Institutional Development Award (IDeA)–eligible states, which are those that historically have had low levels of National Institutes of Health (NIH) funding. The award was made through the NIH Office of Research Infrastructure Programs (ORIP) S10 Instrumentation Program, which supports purchases of commercially available instrumentation to enhance the research of NIH-funded investigators.

Dr. Christopher Cuff—Professor of Microbiology, Immunology & Cell Biology, and Director of Core Resources at WVU—underscored the significance of the NIH supported flow cytometer, the BD LSRFortessa™ (Fortessa) (Figure 1). “Flow cytometry is one of the crown jewels of our core facility activities because of the breadth of the investigators that it serves and the importance that it has in supporting a wide range of investigators funded by the NIH and other sources.”
What Is Flow Cytometry?
Flow cytometers are used to measure specific chemical, physical, or functional properties of individual cells (or particles) as they pass through the instrument in a stream of fluid. Many parameters of a cell can be assessed, such as the molecules on its surface, whether the cell is dividing, whether it is alive, and even the kinds of molecules being produced inside the cell. Most commonly, cells are stained with antibodies, each labeled with a particular fluorescent molecule. During staining, these antibodies recognize and attach to certain proteins on the cells. As each cell passes through the instrument’s lasers, a series of detectors captures the light emitted by the fluorescent molecule. A computer then processes this light into data that investigators can analyze.
Dr. Kathleen Brundage, Director of WVU’s Flow Cytometry and Single Cell Core Facility, offered an analogy to a blood draw. “Blood is made up of red and white cells, and the white cells can be divided further into multiple distinct cell types. You can stain the cells with antibodies that will specifically identify the different cell populations, run them through the instrument, and determine how many cells of each kind you have in your blood.” One clinical application of flow cytometry is customization of chemotherapy for patients with leukemia or lymphoma. Doctors can use a flow cytometer to identify the subtypes of cells present in a patient’s tumor and tailor the patient’s treatment accordingly.
Among the Fortessa flow cytometer’s strengths is its ability to investigate 40,000 cells per second. This allows the study of not only large cell populations but also rare cell populations, even those that may occur at a frequency of 1 in 10,000. “You can actually see such cells and analyze them through all the noise of the other 39,996 cells,” remarked Dr. Cuff. The instrument features a high level of quality control, rigor in data analysis, and reproducibility.
Supporting a Wide Range of Research Projects
The Fortessa at WVU has supported an extraordinarily wide range of research projects united by their focus on individual cells. For many years, flow cytometry primarily was used for immune system research, but as researchers became aware of the technology’s capabilities, it has expanded into more fields. An average of 17 departments from across WVU make use of the Fortessa each year, including those in WVU’s School of Medicine, School of Dentistry, and School of Pharmacy. In each of the last 3 years, approximately 96 individuals from about 50 different laboratories have used its services. Most users of the Fortessa are at WVU; however, every year some users come from smaller colleges, and occasionally, the instrument will be used by researchers from the National Institute for Occupational Safety and Health at the Centers for Disease Control and Prevention.
Since 2015, 48 publications containing data generated on the Fortessa have been published. Research projects have included the following:
- Vaccine development. Investigators have used mouse models to develop new or improved vaccines.1–3
- Cancer research. Many researchers have sought to identify the various cell populations within tumors.4–8 Others are focused on drug discovery and ways to prevent cancer cells from growing by studying the rate of cell proliferation in the presence or absence of a drug or whether the drug induces cell death.9,10
- Mitochondrial function. Investigators have isolated mitochondria—the energy-producing organelles of the cell—to determine their health and how well they function under different circumstances, such as diabetic or obese conditions.11–13
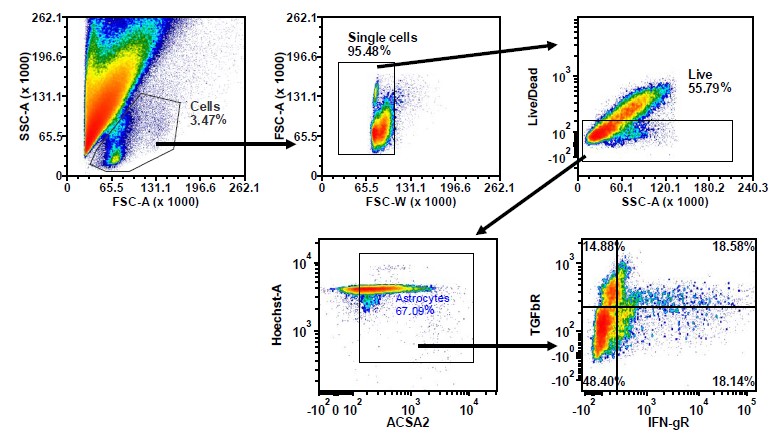
- Stroke and Multiple Sclerosis. Researchers have studied various parts of the brain to see how they respond to stroke induction.14 Others are studying how populations of immune cells and neuronal cells change during the progression of multiple sclerosis (Figure 2).
- Microbiome analysis. Investigators are studying how gut microbiome metabolites affect immune responses. Others are looking at the microbiome inside ticks.
- Dentistry. Researchers are studying the effectiveness of certain treatments at keeping bacteria out of the oral cavity.
- Geology. Investigators are using fluorescent beads to conduct stream/water-flow mapping.
What’s Next for the Fortessa?
As the scope of the research studies supported by the Fortessa has broadened, increasing interest in a wider range of reagents and parameters has followed. Even so, the Fortessa still has room to grow. It currently is configured with four lasers and can detect 17 parameters; it has the capability to add another laser detecting three parameters. Such upgrades will help researchers remain competitive, and Dr. Brundage expects the instrument to function well for another 7–10 years. “The nice thing about flow cytometry is that even instruments that are 10 years old still generate good data,” said Dr. Brundage, who ensures that the instrument is maintained meticulously.
Dr. Cuff highlighted the value of ORIP’s S10 Instrumentation Program. “It is really critical for us to be able to get high-end instrumentation that we need for our researchers to be competitive for their NIH funding.” He also commended the NIH and its Institutes for their support of the variety of research projects that use the Fortessa. In particular, the National Institute of General Medical Sciences has funded a number of significant programs at WVU under the IDeA umbrella, including Centers of Biomedical Research Excellence (COBRE), a program that helps develop new investigators as they strive to obtain R01 funding. Four WVU young faculty hired within the last 4 years have received their first R01 grant using data obtained using the Fortessa. “These investigators really require the best technology that we can offer for them to be successful. The S10 Program is really critical for us to be able to have those kinds of core facilities and instrumentation.” He added that each summer, one to four IDeA Network for Biomedical Research Excellence (INBRE) undergraduates are trained to use the Fortessa. INBRE programs are statewide systems of institutions that expand research capabilities and increase access to biomedical resources.
Dr. Cuff also credited WVU for its role in the continued operation and maintenance of the Fortessa. WVU helps pay for the service contract on the instrument (approximately $34,000 per year). “The S10 Program really is a partnership with the institutions.”
References
1 Boehm DT, Hall JM, Wong TY, DiVenere AM, Sen-Kilic E, Bevere JR, et al. Evaluation of adenylate cyclase toxoid antigen in acellular pertussis vaccines by using a Bordetella pertussis challenge model in mice. Infection and Immunity 2018;86(10):e00857-17. doi: 10.1128/IAI.00857-17.
2 Sen-Kilic E, Blackwood CB, Boehm DT, Witt WT, Malkowski AC, Bevere JR, et al. Intranasal peptide-based Fpva-KLH conjugate vaccine protects mice from Pseudomonas aeruginosa acute murine pneumonia. Frontiers in Immunology 2019;10:2497. doi: 10.3389/fimmu.2019.02497.
3 Varney ME, Boehm DT, DeRoos K, Nowak ES, Wong TY, Sen-Kilic E, et al. Bordetella pertussis whole cell immunization, unlike acellular immunization, mimics naïve infection by driving hematopoietic stem and progenitor cell expansion in mice. Frontiers in Immunology 2018;9:2376. doi: 10.3389/fimmu.2018.02376.
4 Bland CL, Byrne-Hoffman CN, Fernandez A, Rellick SL, Deng W, Klinke DJ. Exosome derived from B16F0 melanoma cells alter the transcriptome of cytotoxic T cells that impacts mitochondrial respiration. The FEBS Journal 2018;285:1033–50. doi: 10.1111/febs.14396.
5 Jones BC, Kelley LC, Loskutov YV, Marinak KM, Kozyreva VK, Smolkin MB, et al. Dual targeting of mesenchymal and amoeboid motility hinders metastatic behavior. Molecular Cancer Research 2017;15(6);670–82. doi: 10.1158/1541-7786.MCR-16-0411.
6 Kozyreva VK, Kiseleva AA, Ice RJ, Jones BC, Loskutov YV, Matalkah F, et al. Combination of eribulin and Aurora A inhibitor MLN8237 prevents metastatic colonization and induces cytotoxic autophagy in breast cancer. Molecular Cancer Therapeutics 2016;15(8):1809–22. doi: 10.1158/1535-7163.MCT-15-0688.
7 Wu L, Amarachintha S, Xu J, Oley F Jr., Du W. Mesenchymal COX2-PG secretome engages NR4A-WNT signaling axis in hematopoietic progenitors to suppress anti-leukemia immunity. British Journal of Haematology 2018;183(3):445–56. doi: 10.1111/bjh.15548.
8 Wu Y, Deng W, McGinley EC, Klinke DJ. Melanoma exosomes deliver a complex biological payload that upregulates PTPN11 to suppress T lymphocyte function. Pigment Cell and Melanoma Research 2017;30(2):203–18. doi: 10.1111/pcmr.12564.
9 Geldenhuys WJ, Nair RR, Piktel D, Martin KH, Gibson LF. The mitoNEET ligand NL-1 mediates anti-leukemic activity in drug-resistant B-cell acute lymphoblastic leukemia. Journal of Pharmacology and Experimental Therapeutics 2019;370(1):25–34. doi: 10.1124/jpet.118.255984.
10 Nair RR, Piktel D, Geldenhuys WJ, Gibson LF. Combination of cabazitaxel and plicamycin induces cell death in drug resistant B-cell acute lymphoblastic leukemia. Leukemia Research 2018;72:59–66. doi: 10.1016/j.leukres.2018.08.002.
11 Kunovac A, Hathaway QA, Pinti MV, Goldsmith WT, Durr AJ, Fink GK, et al. ROS promote epigenetic remodeling and cardiac dysfunction in offspring following maternal engineered nanomaterial (ENM) exposure. Particle and Fibre Toxicology 2019;16(1):24. doi: 10.1186/s12989-019-0310-8.
12 Myers MJ, Shepherd DL, Durr AJ, Stanton DS, Mohamed JS, Hollander JM, et al. The role of SIRT1 in skeletal muscle function and repair of older mice. Journal of Cachexia, Sarcopenia and Muscle 2019;10(4):929–49. doi: 10.1002/jcsm.12437.
13 Shepherd DL, Hathaway QA, Nichols CE, Durr AJ, Pinti MV, Hughes KM, et al. Mitochondrial proteome disruption in the diabetic heart through targeted epigenetic regulation at the mitochondrial heat shock protein 70 (mtHsp70) nuclear locus. Journal of Molecular and Cellular Cardiology 2018;119:104–15. doi: 0.1016/j.yjmcc.2018.04.016.
14 Farris BY, Monaghan KL, Zheng W, Amend CD, Hu H, Ammer AG, et al. Ischemic stroke alters immune cell niche and chemokine profile in mice independent of spontaneous bacterial infection. Immunity, Inflammation and Disease 2019;7(4):326–41. doi: 10.1002/iid3.277.



