Center for Somatic Cell Genome Editing in Nonhuman Primates at the University of California, Davis
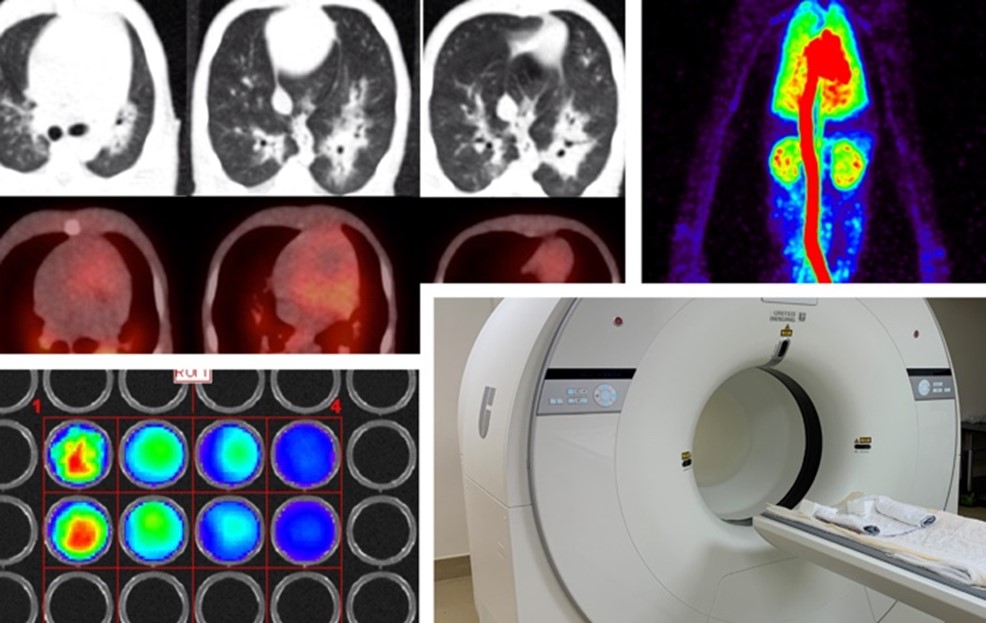
The future of genetic medicine relies on the ability to test therapies safely, efficiently, and ethically in powerful animal models before they reach patients across age groups. Somatic cell genome editing is a revolutionary tool that allows researchers to precisely change the DNA of an organism to correct genetic mutations, offering the potential to cure diseases at their source, even before damage occurs. A focal point of this translational effort is the NIH Center for Somatic Cell Genome Editing in Nonhuman Primates (CSGE, U42OD035737) at the University of California, Davis. The CSGE helps researchers collaborate to accelerate somatic cell genome editing therapeutics to the clinic. Led by Dr. Alice Tarantal, Director and Principal Investigator of the CSGE, the center is a resource that supports NIH-funded investigators nationwide who are interested in pursuing innovative treatments for a broad range of inherited or acquired diseases (Figure 1). “The center’s programs do not focus on a particular disease,” Dr. Tarantal explained. “Rather, our goal is to be at the forefront of cutting-edge research that in some cases can be applied to a range of diseases, thus pursuing groundbreaking scientific discovery.” Therapeutic areas range broadly and focus on multiple tissues, cells, and organ systems, including the heart, lung, blood, muscle, and brain.
Dr. Tarantal emphasized the critical importance of ORIP funding to advancing the field where large animal models, and particularly primate models, are needed. “Considering the steps needed to advance to a human clinical trial, and especially to address diseases in infants and children, a primary objective is safety,” she explained. “Here, it is essential to consider primate models that are most similar to humans from a developmental and organ system perspective.” The physiologic similarities to humans, specifically in immune function, mean that nonhuman primates are invaluable for addressing the safety and efficiency of the approach under consideration.
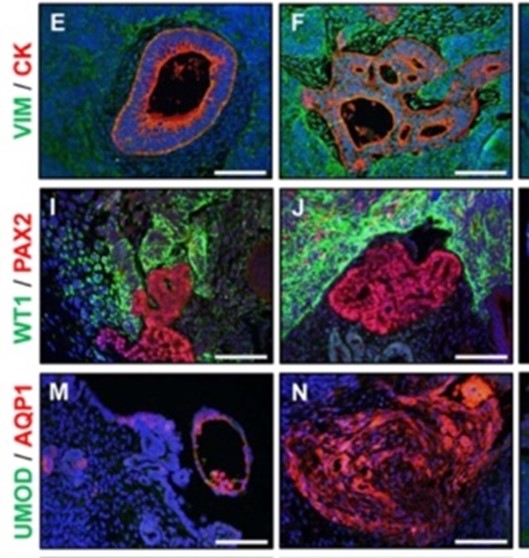
While in silico and in vitro models, such as organoids, are advancing, these tools need to be validated. Three-dimensional organoids improve upon two-dimensional culture systems and can provide human cell–specific information. However, more predictive and informative methodologies are needed to help identify a safety margin when using in vitro methods (Figure 2). Organoids are complex structures that can provide a potentially high-throughput platform for preclinical assessments of efficiency, but organoid outcomes in vitro need to be bridged with clinical measurements in vivo to determine whether they can predict physiologic outcomes. Correlative/parallel testing in vitro and in vivo in the CSGE will help address these questions and assess the efficiency and safety of organoids. The future of biomedical research requires investment in training the next generation of scientists to use these critical model systems alongside new alternative strategies. “It is important that the next generation fully understand the science, as well as the responsibility that comes with using these specialized models, and appreciate their complexity,” Dr. Tarantal emphasized.
The CSGE focuses on a wide array of research needs, from optimizing gene delivery systems and understanding immune responses to exploring how genome editing potentially could impact organ development and function across the lifespan. This program aims to meet the needs of collaborating researchers and help them identify new ways to address vector design, doses, and routes of administration to target specific organ systems or cell types. For congenital disorders that can be detected in utero, prenatal models provide important information for addressing these diseases early, before cell and tissue damage occurs. Dr. Tarantal’s team has shown how editing genes in utero can help researchers understand how diseases originate and progress. “We are addressing new ways to treat early to prevent the onset of disease by targeting the cells or systems, and that requires understanding key developmental events,” Dr. Tarantal shared. This strategy has far-reaching potential for diseases with genetic origins that could be addressed by intervening early.
By providing collaborative opportunities along with access to expertise and essential specialized infrastructure, including innovative in vivo imaging tools, the CSGE helps bridge the gap between preclinical research and human therapies. To advance promising gene therapies by assessing them in nonhuman primates, researchers must first provide compelling preliminary data in other models (e.g., in vitro or small animals, such as mice). Transitioning to primate models requires extensive attention to detail, including designing stringent protocols with careful dosing and deep consideration of vector quality that is comparable to requirements for human trials. Dr. Tarantal’s team routinely guides investigators through this process and offers recommendations to ensure the study design meets essential requirements. As in human studies, in addition to ensuring that antibodies to components of a vector design do not already exist, testing the vector aliquots is essential for optimal experimental outcomes and safety. “A significant amount of work goes into studies with nonhuman primates and ensuring a high level of rigor and reproducibility in parallel with full understanding of the species,” Dr. Tarantal emphasized, sharing her own experiences.
The CSGE also specializes in the use of in vivo imaging modalities in these studies, including ultrasound imaging, optical/bioluminescence imaging, total-body positron emission tomography (PET), and computed tomography (CT). Animals are studied longitudinally, serving as their own controls and thus requiring small cohorts. Imaging modalities, such as PET/CT, can also be applied clinically to provide key insights in real time. Dr. Tarantal emphasized the importance of additional resources provided by ORIP to support the CSGE, such as the High-End Instrumentation Grant Program (S10) for imaging technology and key construction grants (C06) to support essential facilities. The S10 program has enabled the application of state-of-the-art instrumentation essential for these studies, including the use of total-body PET to monitor outcomes noninvasively. “There are several creative ways that we use in vivo imaging to address key questions that investigators may not have considered. We are here to provide the expertise and ensure optimal study design,” Dr. Tarantal noted. Every step in the experimental study is planned with scientific rigor and animal care in mind, as well as future applications in humans.
The CSGE actively coordinates with programs throughout NIH, ensuring that nonhuman primate studies align with broader therapeutic development efforts. Dr. Tarantal routinely pursues new collaborations by searching for investigators working in gene therapy or somatic cell genome editing through the NIH RePORTER. This program also collaborates with other testing centers that explore these studies in other models—including pigs and rodents—allowing a continuum of preclinical evaluation. By integrating across institutes and offering centralized resources, the CSGE streamlines the process for researchers. For many scientists, having access to essential expertise, nonhuman primates across age groups, methods to address new in vitro methods, and associated resources is a game-changer. Additionally, the CSGE also provides expertise to help researchers navigate the regulatory process.
As gene therapy and gene editing advance, the importance of ensuring safety and reproducibility cannot be overstated. The CSGE takes a collaborative approach, meaning that every project it supports is designed to address these concerns from the start—whether through optimized vector design and delivery, understanding of the potential for immune responses, or long-term monitoring via in vivo imaging. “Our goal is to ensure we are addressing these questions efficiently … NIH funding is key for any newly approved technologies and therapeutics to advance from the bench to the bedside and back when needed,” Dr. Tarantal emphasized. With ORIP’s continued support, the CSGE is charting a course toward more effective, reliable, safe, and ethical treatments, turning scientific breakthroughs into clinical realities.
ORIP continues this initiative for large animal testing centers for the evaluation of somatic cell genome editing tools by building on the success of previous funding through the NIH Common Fund Somatic Cell Genome Editing Phase One Project, supporting small (rodent) and large (nonhuman primate and swine) animal testing centers to evaluate in vivo genome editing technologies. These centers collaborate with NIH-funded investigators to assess safety and efficacy, manage animal cohorts, and accelerate the translation of genome editing tools into human treatments. ORIP continues to fund initiatives and create resources for the entire biomedical community.



