Cell Ablation System in Zebrafish Yields Insights on Cellular Regeneration
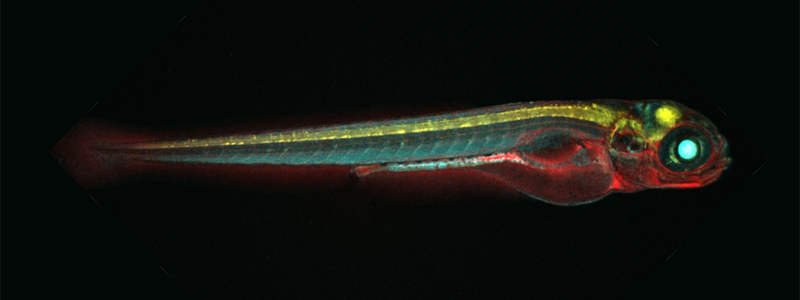
The zebrafish (Danio rerio) has been used by researchers as a model organism for human disease for decades. In recent years, however, the zebrafish has gained considerable attention for its regenerative capacity (Figure 1). For example, the zebrafish possesses the ability to regenerate cardiac muscle cells after the removal of tissue from the heart’s ventricle.1 Researchers are interested in better understanding regeneration in zebrafish and applying the lessons learned to the development of therapeutics for many human diseases.
Dr. Jeffrey Mumm, an associate professor of ophthalmology at the Wilmer Eye Institute, Johns Hopkins Medicine, is interested in how zebrafish respond to the destruction of different cell types in their body. The findings from Dr. Mumm’s research could be applied to many human conditions, including such neurodegenerative disorders as Alzheimer’s and Parkinson’s diseases. “[Tissue] regeneration is astounding and cool,” Dr. Mumm emphasized. “But cellular regeneration is more applicable to degenerative diseases associated with or triggered by the loss of individual cell types.”
Dr. Mumm first became interested in cellular regeneration as an undergraduate student at The University of Iowa. He explained that the potential for therapeutic regeneration likely lies within the human genome. To uncover this information, researchers must better understand the cellular and molecular mechanisms regulating regenerative potential in each type of tissue.
“Genetically, there’s no impediment to humans’ mounting regenerative processes; however, tapping into our full regenerative potential will require novel therapeutic strategies,” Dr. Mumm stated. “The entire field of medicine is about overcoming our own biological limitations. In the grand scheme of things, I believe [cellular regeneration] is no different.”
To facilitate cellular regeneration studies, Dr. Mumm adapted a conditional targeted cell ablation system to zebrafish (Figure 2).2 This system works by chemically eliminating cells that transgenically express a bacterial nitroreductase (NTR). The initial iteration of this system used an NTR enzyme from Escherichia coli, encoded by the nfsB gene.3 The system was useful for investigating both the function and regenerative capacity of different cell types in zebrafish and other model systems, but the high concentration of prodrug (i.e., the compound that is converted into the active drug following administration) that is required for effective ablation was problematic.
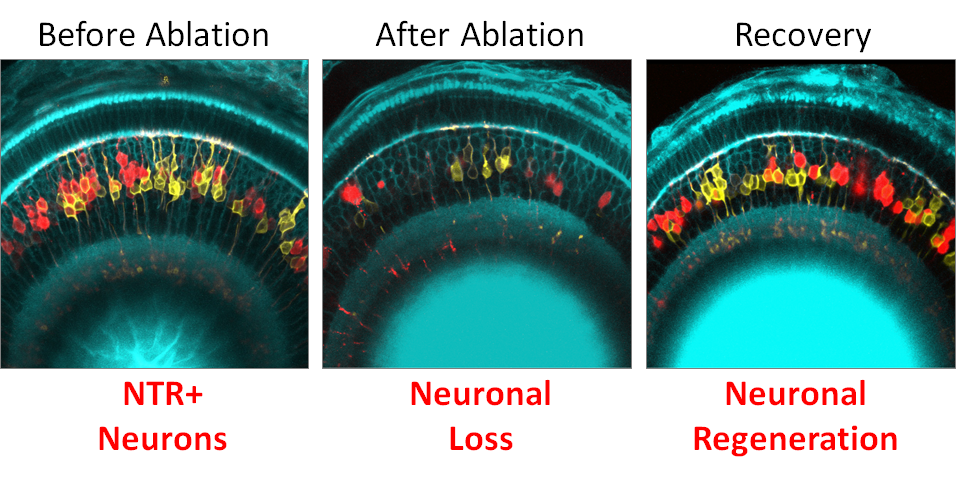
With the support of an ORIP grant (R01OD020376), Dr. Mumm has begun collaborating with Dr. David Ackerley, professor of biotechnology at Victoria University of Wellington, to identify and engineer improved NTR variants that facilitate ablation at lower concentrations of prodrug. The team recently succeeded in engineering an NTR from Vibrio vulnificus, a marine flesh-eating bacteria, that displays 100-fold greater prodrug conversion efficacy. This “NTR 2.0” variant enables new animal models and novel cell ablation paradigms. For instance, long-term, low-dose prodrug exposures now can be used for sustained ablation, mimicking chronic cell loss diseases in normally regenerative species, such as zebrafish. Dr. Mumm underscored the importance of such paradigms to understand how cell regeneration is regulated at the molecular level, thus supporting the development of regenerative therapeutics for a host of chronically progressive degenerative diseases.
To facilitate the discovery of factors that regulate cell regeneration, Dr. Mumm adapted high throughput screening methodologies to zebrafish. With this approach, his team can track cellular fluorescent reporters—and quantify cell numbers—in individual zebrafish larvae placed in 96-well plates.4 Dr. Mumm explained that this novel technique enables high-throughput screens in living models for factors that promote cell survival5 and accelerate cellular regeneration.
With the support of an ORIP shared instrumentation grant (S10OD026909), Dr. Mumm now is expanding the capabilities of his in vivo screening system. A laser light-sheet confocal microscope adds high-content three-dimensional imaging capabilities, and a swappable microfluidics system will allow large-scale screens across a broad array of whole-organism and three-dimensional cell culture models. Dr. Mumm and colleagues posit that cross-species drug discovery projects enabled by the new screening platform will allow the identification of highly conserved pharmacological targets and pathways. The zebrafish might hold the key to translating regenerative therapeutics to the clinic and restoring quality of life to patients.
References
1 1 Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190. doi:10.1126/science.1077857.
2 Curado S, Anderson RM, Jungblut B, et al. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236(4):1025–1035. doi:10.1002/dvdy.21100.
3 White DT, Mumm JS. The nitroreductase system of inducible targeted ablation facilitates cell-specific regenerative studies in zebrafish. Methods. 2013;62(3):232–240. doi:10.1016/j.ymeth.2013.03.017.
4 Wang G, Rajpurohit SK, Delaspre F, et al. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic β-cell mass. eLife. 2015;4:e08261. doi:10.7554/eLife.08261.
5 Zhang L, Chen C, Fu J, et al. Large-scale phenotypic drug screen identifies neuroprotectants in zebrafish and mouse models of retinitis pigmentosa. bioRxiv. 2020. doi:10.1101/2020.03.26.010009.



