Profile of a Veterinary Anatomic Pathologist and Researcher: Heather Shive, D.V.M., Ph.D., DACVP
Since her childhood, Dr. Heather Shive (Figure 1) has been interested in working with animals. This interest, combined with a love of scientific inquiry, led her on the path to becoming a Doctor of Veterinary Medicine (D.V.M.) who specializes in anatomic pathology, as well as a cancer researcher. Dr. Shive began her research career in NIH’s Comparative Biomedical Scientists Training Program (CBSTP) and—after establishing herself as a talented young investigator outside the NIH ecosystem—was recruited back to NIH to continue her research program and serve as co-director of the CBSTP.

Dr. Shive received her bachelor’s degree from the University of Arizona and her D.V.M. from the College of Veterinary Medicine at North Carolina State University. She then spent 2 years in a residency program in anatomic pathology at the College of Veterinary Medicine at Texas A&M University. Dr. Shive explained her interest in pathology: “I originally went to veterinary school thinking that I was going to go into general practice, as many people do. When I was a second-year veterinary student, that’s when we first had our pathology courses. The ideas that the courses introduced me to—in terms of thinking about medical processes and how specific pathways underlie genetic conditions and so forth—were really new and eye-opening to me. I’m a person that really likes puzzles and mysteries, and pathology is an amazing way to gain the deepest possible understanding of medical conditions and disease processes.” She also noted that she became deeply interested in cancer research during her residency. “I realized in the course of that residency that scientific inquiry is really important to me in terms of being engaged in the work that I do. I felt that diagnostic work alone would not be enough.”
Dr. Shive applied to the CBSTP to explore her interest in research. She was accepted as a Graduate Partnership Programs fellow and completed her Ph.D. research under the training of Dr. Dennis Hickstein in the Experimental Transplantation and Immunology Branch of the Center for Cancer Research (CCR) at the National Cancer Institute (NCI) and in partnership with the University of Maryland (UMD). During this time, Dr. Shive achieved board certification in anatomic pathology from the American College of Veterinary Pathologists.
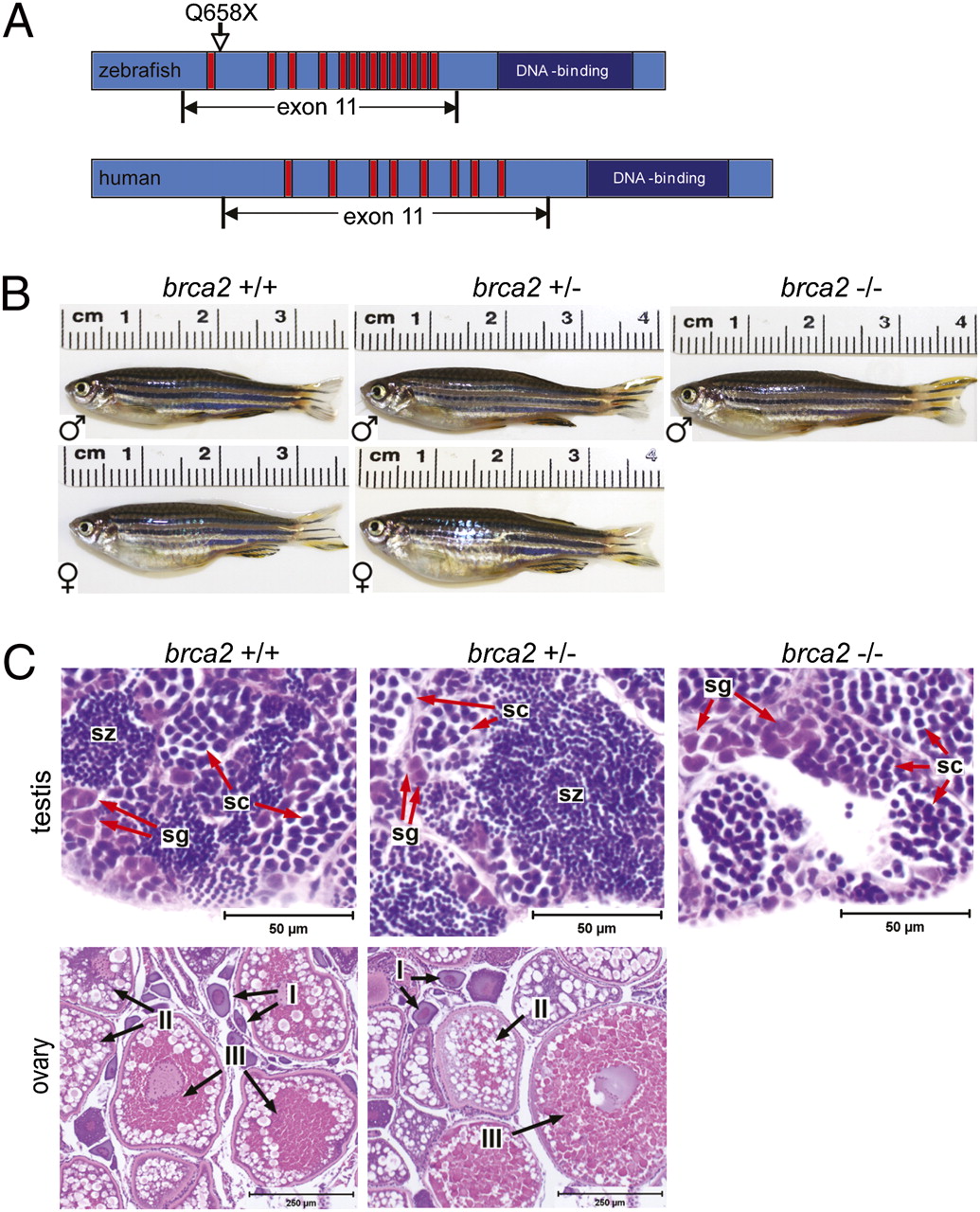
Dr. Shive’s Ph.D. research focused on the role of Breast Cancer Gene 2 (BRCA2) in normal development and tumorigenesis. Mutations in BRCA2 are associated with an increased risk of developing breast and ovarian cancer, and the BRCA2 protein is known to function in DNA repair; however, in-depth studies have been limited by the early embryonic lethality observed in mice lacking BRCA2. To circumvent this challenge, Dr. Shive turned to zebrafish as a model organism and showed that fish carrying a homozygous brca2 mutation (which is similar to a common BRCA2 mutation in humans) survive to adulthood but exhibit profound defects in the development and maintenance of reproductive tissues (i.e., failure to develop ovaries in juvenile fish, development of testicular neoplasia in adult fish) (Figure 2).1 She also determined that combined mutations in brca2 and tp53 (another important tumor suppressor gene in humans and animals) significantly increased cancer risk in zebrafish; similarly, human cancers associated with inherited BRCA2 mutation frequently develop TP53 mutations.2
Dr. Shive is enthusiastic about zebrafish as a model organism. She was drawn to the unique technologies that can easily and inexpensively be incorporated into zebrafish research. Zebrafish can be used to address questions that are difficult or impossible to evaluate in mammalian models. Approximately 70 percent of human genes have at least one discernable zebrafish ortholog,3 and many of these genes have been characterized and shown to function in similar ways in fish and humans. Dr. Shive warns, however, about the need to constantly consider which model systems (animal and otherwise) are most suitable to address various scientific questions. “It’s important,” emphasized Dr. Shive, “for all of us researchers to be thoughtful about the models that we choose and to know when the model that we’re currently using needs to be traded for something else.”
Dr. Shive remained at NCI as a staff scientist in the CCR’s Experimental Transplantation and Immunology Branch after graduating from the CBSTP in 2010 with a Ph.D. granted by UMD. She continued promoting zebrafish as an excellent model for human cancer as she transitioned to faculty positions at North Carolina State University and then The Ohio State University. In 2016, Dr. Shive was awarded an NIH Special Emphasis Research Career Award (SERCA) K01. The ORIP Division of Comparative Medicine funds SERCA awards in pathology and comparative medicine to provide support for veterinarians with experience in animal and comparative research to become independent investigators. SERCA K01 awards focus on in-depth mentored research experience in basic or clinical scientific disciplines and provide up to 5 years of support for individual grantees.
Dr. Shive and her colleagues had previously demonstrated genetic similarities between human and zebrafish BRCA2-associated cancers (including a collaborative role for the tumor protein 53 [tp53] in BRCA2-associated carcinogenesis). Dr. Shive’s SERCA K01 application proposed using the brca2-mutant zebrafish model to identify novel, conserved mutations that contribute to carcinogenesis. She has published several papers using the zebrafish model, including an immunohistochemical analysis of zebrafish with mutations in both brca2 and tp534; a study that linked brca2 mutations with tumor diploidy and with poor survival outcomes in cancer-bearing females5; and a study showing that maternally provided brca2 RNA is required for early embryonic development and that adults without brca2 exhibit aberrant cellular proliferation in tissues that are predisposed to cancer development.6

“Getting the K01 was an amazing addition to my abilities as a researcher. It made it possible for me to devote much more time to the research that I wanted to do. It made it possible for me to do experiments that I would not have been able to do otherwise. It also opened a lot of doors for me in terms of making connections with other people, both within my university as well as others,” she explained. “Having received a K01 award, people take more of an interest in your science and more of an interest in you as a young investigator.” The K01 award led Dr. Shive to opportunities like involvement in a grant-writing club with other K01 investigators at North Carolina State University and likely led to her being asked to serve on an NIH grant review committee.
While conducting research at The Ohio State University, Dr. Shive was contacted by Dr. R. Mark Simpson, the founder of the CBSTP, with a proposal to return to NIH and co-direct the program. Dr. Shive admits that it was a difficult decision, but the opportunity to be involved in research and training efforts at NIH was too exciting to decline. In 2022, she rejoined the CCR as a staff scientist and co-director of the CBSTP. “Coming back to help lead the program that was so important for me in my own career development—I’m really happy to be here and to be part of this program,” explained Dr. Shive. “Doing research within the NCI community is amazing. It was amazing as a graduate student, and it’s amazing now.”

As part of her responsibilities, Dr. Shive serves as a mentor to CBSTP fellows (Figure 3), as well as veterinary students who join NIH for summer research internships (Figure 4). She pointed out that younger veterinary students appear to be more aware of career possibilities beyond being general practitioners. When mentoring younger students, Dr. Shive advises them to persevere against challenges and to not be afraid to experiment with new model systems and technologies. As Dr. Shive notes, “the excitement when you get something to work for the first time … it’s magic.” She also emphasizes flexibility in one’s career path. “It’s okay to change your mind, and I think my own career decisions and career pathway are probably an example of that,” she pointed out. “I think it’s important to keep an open mind and to pay attention to what you’re good at and what you really enjoy.”
For additional information on the SERCA K01 Scholars Program, see the ORIP SERCA Guidelines.
References
1 1 Shive HR, West RR, Embree LJ, et al. brca2 in zebrafish ovarian development, spermatogenesis, and tumorigenesis. PNAS USA. 2010;107(45):19350–19355. doi:10.1073/pnas.1011630107.
2 Shive HR, West RR, Embree LJ, et al. brca1 and tp53 collaborate in tumorigenesis in zebrafish. PLoS One. 2014;9(1):e87177. doi:10.1371/journal.pone.0087177.
3 Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi:10.1038/nature12111.
4 White LA, Sexton JM, Shive HR. Histologic and immunohistochemical analyses of soft tissue sarcomas from brca2-mutant/tp53-mutant zebrafish are consistent with neural crest (Schwann cell) origin. Vet Pathol. 2017;54(2):320–327. doi:10.1177/0300985816669406.
5 Mensah L, Ferguson JL, Shive HR. Genotypic and phenotypic variables affect meiotic cell cycle progression, tumor ploidy, and cancer-associated mortality in a brca2-mutant zebrafish model. J Oncol. 2019;9218251. doi:10.1155/2019/9218251.
6 Kouprianov VA, Selmek AA, Ferguson JL, et al. brca2-mutant zebrafish exhibit context- and tissue-dependent alterations in cell phenotypes and response to injury. Sci Rep. 2022;12(1):883. doi:10.1038/s41598-022-04878-9



